`
To be continued in "Expanding Upon: Via pH & Salinity "DMT" Converts "Amino Acids" to LIVE Major Species Configuration Capable of Consciously Coordinating Dynamic "Reversal" of NAc, mRNA Protein Systems & Pt Nanotubes!"
Downloadable PDF of this publication which has long exceeded it's original names and content establishing the precise locations via which N,N-Dimethyltryptamine (DMT) [1] complete bio-molecular circuitry between an "ancient RNA-binding motif" in Sm Domain [2] and otherwise unexplained unusually placed C terminal extension Sml1 Complex [3, 4, 5] associated with temporary yet critical reconfiguration of fully activated standard proteinogenic "amino acids". Quantum computations have recently proven possible basics actions confirming that DMT (& similar substances) complete the amino set (particularly within hydrogen saturated biochemically diverse environment [18]) along with additional studies demonstrating “stretching motions of the five-member ring coupled with NH bending” [87] support the hypothesis that DMT possesses conscious stereochemical facility to complete the circuitry of a hyper divergent secondary amino configuration. Such temporary maintenance configuration provides dominant live amino's full control of complex intermolecular processes including transitional metal nano structures and other processes associated RNA gene splicing & a range of intermittent yet critical actions including many relating to mRNA proteins. In addition to the above, numerous reports confirm evidence of mirrored negative positive circuitry system within the AMPA glutamate receptors (AMPARs) which are associated with dysfunctional RNA editing that additionally and also include an array of auxiliary subunits offering complex range of possible configurations [36, 37]. This provides clearer evidence of reverse configuration potentially requiring DMT to merely facilitate the reversal of standard amino acids basic actions, in addition to countless combinations of configurations dependant upon consciousness as critical component of DMTs stereochemically molecular facility.
The Following United States of America Patent Publication (114pg Downloadable PDF directly below)...
Provides comprehensive scientific evidence confirming the countless systems and pathways, running in conjunction with above via which the regular consumption of DMT (and similar "substances") are capable of preventing virtually every disease known to mankind [38]. However, the patent does not appear to include consideration of scientific evidence regarding the consciousness of Amino Acids particularly in conjunction with the secondary configuration facilitated by DMT (etc) which is of critical relevance to the potential dangers associated with their capitalistically inspired "invention" designed to impair consciousness in conjunction with activating the hyper-divergent amino configurations the genetic systems involved. In addition to clearly complex oversimplified example of the AMPARs including both a reversible mirror system accompanied by comprehensive options for dynamic "duel" (or combined) configurations - such complexities are drastically increased by their interrelationships with the systems listed in the following section of the US Patent publication above....
The term mRNA is only included in the written sections [0078], [0093] and [0166] of the Patent document. However, the complex interrelated pathways, proteins and context this is predominately in reference are strongly associated with mRNA all of which upon enquiry include dysfunction associated with countless diseases and evidence of alternate pathways that cannot be scientifically explained by the ridged rules of standard configurations of "amino acids” indicating an alternate configuration. Whilst evidence supporting a highly divergent secondary configuration has so far consistently been identified across all systems Universal Asylum has presently briefly explored - it is only recently that technological advancements have produced evidence of one of countless examples supporting how such an alternate configuration is possible.
The fact that someone was retarded enough to create a legal document full of scientific evidence mapping out the pathways such systems were obviously naturally designed to work together just fine with appropriate sustenance (neglecting such discussion) - whilst claiming ownership of inventing a way of advancing and retarding them at the same is fucken frightening. Especially given this was filed in 2020 during the pandemic.
Whilst this publication has a strong emphasis the interrelationship between DMT, pH, salinity and consciousness supporting the proposition of a secondary divergent configuration of "amino acids” capable of harmoniously facilitating the complex interrelationships of and between the processes and pathways above and more - It should be noted that such discussion is intended to inform consideration of analysis of samples collected throughout Universal Asylums Metabolic Implications of "Psychedelics / Forbidden Fruit" upon DRUG Saturation during which the administration of naloxone to a Suboxone saturated human test subject whilst under the influence of Psilocybin, Mescaline and Ayahausca was consistently unable to induce precipitated opioid withdrawal. Additionally, it is written from the perspective of a Social Worker with enough informal experience and scientific understanding to conceptualises and undertake research of a level otherwise pushing the boundaries of my professional scope in exploring more technical considerations of speaking to sample analysis and my theoretical understanding of the subject. Therefore, the following is not as well structured or worded as I would like and on occasion potentially makes statements it later challenges mapping out and exploring potentially relevant factors.
Science is yet to reach a consensus on the origin of life, consciousness or "amino acids" which are recognised as sharing intimate coevolutionary relationships with RNA viruses for 4 billion years during which there is not shortage of evidence of amine's significant influence over protein development [25, 26]. Additionally rapidly increasing evidence of universally symbiotic interrelationships between amines and all forms of life, in conjunction with all natural elements included with existence. The most obvious plausible explanation for this compatible with countless scientifically unresolved phenomenons of life such as migration, planetary rotation, aquatic directional pull, etc given evidence of their 'racemic" mirrored configuration in space [59] - is
that Indolamines are the conscious co-creators and/or perhaps even the original procreators of life on earth and perhaps even itself. Whilst this suggests they are a component of a collective consciousness and amongst or perhaps even exclusively the elusive universal ancestor(s) of the evolutionary tree of life hidden in plain sight due to DMT facilitating such a complex temporary "duel" configuration capable of breaking pretty much all of the rules. However, possession of consciousness doesn't necessarily resolve whether they are themselves the producers or products of consciousness. Regardless, based on the available evidence unencumbered indolamines appear a damn sight smarter than the 8 billion allegedly intelligent dumb cunts so afraid of dying most never truely live whilst fucking the world up for everything else. Unfortunately, scientific discussion of the emergence of life on earth frequently fails to serve itself or amines justice by regurgitating the "building blocks of protein" hypothesis after having otherwise debunked with discussion of interactive responses to ecological conditions only possible if amines possessed "consciousness" initially likely to have required greater dependance upon what for many species has evolved to severe as a secondary configuration.
The N-methyl-d-aspartate receptors (NMDAR) are nowhere near as clear as the AMPAR but some interesting things immediately stand out particularly the pH in the Nr1 subunit discussed later [39]. pH and salinity will become increasingly relevant in due course.
According to linked Screenshot of the Weizmann Compass which I will quote the most important of encase the screen shot is unclear & at a quick glance of the titles of his Dr. Italy Helevy's publications which include so many crossovers I currently don't have access to or time to thoroughly explore looking for a better direct quote!...
"Dr. Itay Halevy of the Weizmann Institute’s Department of Earth and Planetary Sciences, together with Dr. Aviv Bachan of Stanford University, has developed a model of how seawater pH has changed since ancient times. Integrating data and existing models of the chemical makeup of the Earth’s atmosphere, oceans, and crust, the scientists’ new model identifies the main “culprit” behind seawater’s changing chemistry: the gradual decrease in atmospheric CO2 levels in response to an increase, over eons, of the brightness of the Sun.
According to the model, which was reported in Science, the oceans of the distant past were mildly acidic, then, over eons, acquired more alkaline pH levels.
The study found that three to four billion years ago, the pH of ocean water was acidic in the range of 6.0 to 7.5—somewhere between that of milk and human blood—as opposed to the more recent, relatively alkaline values of 7.5 to 9.0. The findings help clarify the chemical conditions under which primitive life emerged and thrived in the early oceans - and also help us understand the dynamic changes that contributed to the modern ocean’s chemical balance."
pH & the N-Methyl-D-Aspartate receptors (NMDAR) NR1 subunit 3-4billion years later?
"NR1 subunit
The mRNA of the NR1 subunit begins to be expressed in the brain of the rat embryo as early as day 14 of gestation, and its levels gradually increase until 3 weeks after birth.14 There are 8 processing variants for NR1 mRNA (NR1-1a/4a y NR1-1b/4b), which differ among each other according to the presence or absence of a sequence of 21 amino acids (N1: exon 5) in the N-terminal region (Fig. 4), and differential processing of exons 21 and 22 which provokes changes in sequences in the C-terminal region (units C1, C2 and C2′)15,16 (Figs. 5 and 6). The N1 region is important for the regulation of channel properties, since it alters its sensitivity to spermine, pH, and zinc.17 In isoforms containing the N1 exon, neither polyamines nor Zn+increase stimulation by Glu, which may be due to it being a cation and its repulsion of the exon. The presence of the N1 exon is also linked to properties such as receptor affinity for agonists and their sensitivity to the antagonists APV ((2R)-amino-5-phosphonovaleric acid), CPP, 7-CK and MK-8 01. NMDAR sensitivity to pH is determined by the presence of exon 5. At physiological pH, receptors that include this variant are activated completely, while receptors that lack exon 5 are partially inhibited.18,19"r [39]. Also of potential relevance given life started out in the ocean: initially cited via Autry AE, Monteggia LM (2012) "there is also evidence that BDNF may directly activate voltage-gated sodium channels to mediate rapid depolarization of target neurons (Blum et al., 2002)" [40]. This is particularly relevant considering recent scientific revelations regarding sodium and water [43] in a study claimed to force text books to be rewritten identifying variations in the arrangement of molecules at the surface of saltwater creating a far complex and dynamic environment [44].
Chart reference [43]
Such variation in water polarisation and the negative/positive charged arrangement of salinity at the interface between water and air is likely to be of significance to the complex dynamic states facilitated by DMT, etc. Particularly given quantum computations recently proved stereo-inversion possible via appropriate placement of a single water molecule within a gaseous pocket in an otherwise saturated environment [6, 18]. Whilst complex to speak to in general across multicellular lifeforms such as plants and animals, given the well scientifically documented roll of DMT in regulation of inflammatory systems likely to require coordination between liquid and gaseous states - it is reasonable to suggest that DMT is capable of applying salinity dependant reversible charge as required within the saturated environments of living multicellular organisms. However, perhaps the latter is more appropriately worded "the multicellular organisms within which DMT etc lives". Nevertheless, in addition to the broader physical evidence supporting the proposal of a dynamic secondary amino configuration capable of facilitating the phenomenal results of the experimental research in question - this has certainly added to the complexity of the circuitry of premature excitement over basic hydrogen bonds and their potential interrelationships with dysfunctional hydrolytic splicing [60]. This may also be of particular relevance to "Mast Cells" as "Cromolyn sodium is a mast cell stabilizer that is thought to act on both chloride channels and signaling proteins in the cell membrane to inhibit degranulation" [62]. The interelationship between Mastcells, pH [65, 66], and salinity [67] is also of great interest likely to play a role in the secondary amino configuration.
Image source [63].
Mast cells are said to be found in every organ except the CNS from bone marrow where they derive to connective tissue often in higher accumulation in places exposed to bacteria such as skin and respiratory, gastrointestinal and around blood-vessels and lymphatic systems. Mast cells have major roles in healing and are associated with CD4+ T Cells and mTOR [64]. Once mature mast cells are not thought to circulate leave their specific area under normal circumstances. However, this results of the medical experiments in question warrant investigation in this area. Mast cells are of great interest in current analysis of mucus samples due to their potential to shed light on the respiratory and gastrointestinal tracts roles as a therapeutic channels of expelling pathogens or other components of sickness enhanced whilst "under the influence" of DMT etc. Mast cells also have significant roles in the regulation of inflammation [62].
Additionally at a glance "Brain derived neurotrophic factor (BDNF) is the most prevalent growth factor in the central nervous system (CNS). It is essential for the development of the CNS and for neuronal plasticity." [40]. BDNF is of potential relevance to considering analyses of blood-plasma samples collected via Universal Asylums Experimental Research Metabolic Implications of "Psychedelics / Forbidden Fruit" upon DRUG Saturation - I could have never imagined was going to prove such an evolutionary learning experience... "During opiate withdrawal, BDNF mRNA expression increases by 2- to 3-fold up to 3 days after opiate removal (Numan et al., 1998). This dramatic increase corresponds to enhanced TrkB receptor expression within 6 h of withdrawal (Numan et al., 1998)." [40]. Particular in respect to previously identified crossovers between viruses and opiods in relation to T-Cells in the publication How N,N-Dimethyltryptamine Works?: DMTs Biophysiological Hydrogen Bond with H2O Supporting Multicellular Life & Hyper Generalised Cellular Divergence, Intracellularly, Extracellularly & Transcytosis. Such overlaps are predominately associated with Th17, Th1, CD4+ & Dendric cells as components of the mTOR system depicted in this illustration from [48].
Illistration and description source [48]
The Nucleus Accumbens (NAc) appears to be a key and/or perhaps central component or primary interface for the secondary amino configuration supported by N,N-Dimethyltryptamine (DMT) etc. According to Castro & Bruchas (2019) [56] there is consistent evidence that the rules do not apply and/or are reversed in functional logic in relation to the NAc's medial shell. In addition to neuro-peptidal, glutamate, phosphorylation, and opioid and amphetamine actions [56] the high density of dendritic spines, accounting for 90–95% of all neurons in the striatum surrounding the NAc yet appear mysteriously inactive [57] is of great interest. Such interest is due to the fact that "pH homeostasis in neurons plays crucial roles in normal synaptic functions. It is found that the Na+/H+exchanger NHE5 is targeted to the synapse on neuronal activation, regulates the synaptic pH, and controls the morphology of dendritic spines" which undergo dynamic structural changes in response to neuronal activation [2]. However, this publication proposes that it is in fact DMT's secondary indolamine configuration responsible for consciously regulating the pH and salinity via intermolecular stereochemical processes as a key component of the complex mechanisms associated with evidence confirming a divergent secondary system involving dendritic spines [58] associated with pH [2, 56, 57, 58].
Additionally, to provide further context directly quoting Universal Asylums introduction to earlier work "In providing a brief summery leading into deeper analysis. According to Liang et al (2016) T cells, particularly CD4+T helper cells Th1 and Th17 and Dendritic cells are the most potent antigen-presenting cells and play a critical role in supporting innate and adaptive autoimmune system functionality. Opioids are recognised to cause major disruption to the CNS system including neuro-inflamation, dis-regulation of immune systems with significant impacts regulatory T cells, particularly CD4+ T, Th17 and Dendritic cells and including anti proliferative, cellular exhaustive and pro-apoptotic features (Plein & Rittner 2018) [49] with RNA gene sequencing confirming their impacts on gene expression (Mikati 2023 & Sacerdote 2013) [50]. In human bone marrow, δopioid receptor mRNA express at a low level in immature dendritic cells, conversely, at a high level in mature dendritic cells which is an area of congestion which is particularly painful under “regular” conditions. Whilst frequently describe as immune-modulators... available evidence suggests that opioids cause cellular dis-regulations with various pros and cons under different conditions. Chronic inflammatory diseases are the most significant cause of death in the world. The World Health Organization (WHO) ranks chronic diseases as the greatest threat to human health (Pahwa R, et al,. 2023) [51]. A balanced combination of pro-inflammatory and anti-inflammatory mechanisms would facilitate viral clearance and immunity to reinfection, with minimal damage to host tissues (Rouse & Sehrawat) [52]. DMT and similar substances are scientifically recognised as activating particularly CD4+T helper cells Th1 and Th17 and Dendritic cells, supporting cellular proliferation, regulation of inflammatory systems with antiviral, anti tumour, anticancer and neuroregenerative actions (Szabo et al 2014 [53], Szabo & Frecska 2016 [54] & Rudin 2023 [55])."
"The activation of the mTORC1 pathway is coupled with diet mediated changes in the concentration of amino acids. Interestingly, the mechanism of sensing the amino acids through mTORC1 with the help of Rag GTPases as essential components of mTORC1 signaling was one of the groundbreaking discoveries in the field of mTOR signaling. This synergistic effect facilitates effective T-cell activation by upregulating mTOR function" [40]. This is of great relevance to the question of consciousness and control of such critical complex interdependent systems and processes as one of the primary topics of this publication. According to Panwar, Vivek & Singh, et al (2023) "The mTOR belongs to the class of evolutionarily conserved threonine and serine kinases which recognize and incorporate a variety of extracellular and intracellular signals to maintain cellular homeostasis and metabolism 1–4 The name mTOR was obtained from rapamycin isolated from a soil bacterium in 1970 on Rapa NuFurther, the structural elucidation of the rapamycin revealed 14–16 membered lactone rings and reduced saccharide substituents... Mechanobiology of mTOR unfolds it as a complex protein kinase intricated with multi element complexes via its communication network with other proteins." [41]. [42]. mTOR has also been identified to have pH dependant variabilities between mTORC1 and particularly mTORC2 the latter of which was said to be inhibited [48]. However, at the right pH it is likely that this isn't the case as a component of reconfiguration of amino regulation including their interrelationships with mTORC2.
The following is of direct relevance to the above in both standard and secondary configurations. However, is also of diect relevance to later discussion of the prospect of additional platinum Pt and/or yttrium Y nanotubes particularly in secondary configurations.
"Fig. 4. Significance in mature neurons of the three major pools of α-tubulin resulting from the detyrosination/re-tyrosination cycle. Schematic representation of detyrosinated (deTyr, green), tyrosinated (Tyr, magenta) and Δ2 (red) microtubules whose orientation is represented with a "+" at the microtubule +end. Effectors of the cycle, such as molecular motors (with their specific neuronal cargos) and CAP-Gly +end proteins, are represented in the color of the microtubule to which they are sensitive. Inbox 1: dendrites contain mixed-polarity microtubules composed of Tyr tubulin at their outer region and deTyr and ∆2-tubulin at their inner region; deTyr-sensitive kinesins-1 and 2 transport GABA(A), AMPA and NMDA receptors [129], [130], [135]. Inbox 2: Tyr microtubules from the dendritic shaft can transiently enter into dendritic spines. Tyr-sensitive kinesin-3 transport synaptotagmin vesicles into the spines [136]. Tyr-sensitive CAP-Gly proteins could have the following roles in spines: CLIP-170 and p150-Glued could interact with growing end of microtubules [57]; CLIP-170 could increase microtubule dynamics and favors actin polymerization[26], [137]; P150-Glued could initiate the retrograde dynein-dependent transport out of the spines [54]. Inbox 3: axonal microtubules have uniform polarity and are mainly composed of stable deTyr and ∆2-tubulins. Tyr-sensitive kinesin-5 transport microtubules and kinesin-3 transport vesicles [48], [127]. DeTyr-sensitive kinesin-1 transports mitochondria and TrkB [126], [133]. Inbox 4: axonal boutons are hotspots of microtubule remodeling and delivery of synaptic vesicles driven by the Tyr-sensitive kinesin-3 [127]. Kinesin-3 also transports TrkB at the axon distal part [126].
Figure adapted from[114]." Figure borrowed from [69].
After over 4 billion years, 20 amino acids have remained common to all forms of life [27] with evidence suggesting this was initially as few as 10 [26]. According to CHEBI (2024) (CHEBI:83813) - there are 23 protonated amino acids. These include 20 in the standard genetic code, 9 of which: phenylalanine, valine, tryptophan, threonine, isoleucine, methionine, histidine, leucine and lysine are considered essential whilst the remaining 11: alanine, arginine, asparagine, aspartic acid, cysteine, glutamic acid, glutamine, glycine, proline, serine and tyrosine allegedly aren't essential. Plus selenocysteine and pyrrolysine as 21 & 22 which apparently can be genetically incorporated in translation (suggesting they are at least relatives) and finally 23 N-formylmethionine which appears genetically unidentified or undisclosed at this point in time. Despite the last 10 letters of N,N-Dimethyltryptamine confirming it is an indoleamine known for it's most significant direct interrelationships with tryptophan, likely to include complex combinations of both anabolic and catabolic processes under different conditions of critical significance to the coevolution of all life on earth - for political reasons DMT is classified as an illicit substance rather than an essential yet intermittently required member of it's amino family.
Maguire, et al pointed out 20years ago "the deletion of as few as three amino acids at the N terminus of L27 leads to an impaired PT (peptide) activity of E. coli ribosomes" [7]. This becomes particularly relevant when those three aminos are critically essential to facilitate periodically required fully charged equalibrium enabling Tryptophan and Tryptamine shared control over deviating from the typical amino configuration in coordinating complex combinations of multidirectional macrocyclic intermolecular interactions between protonated amines of both L and D amine variants. A great deal of evidence indicates an "unknown neurotransmitter" of male charactor, who's absence is associated with unhealthy imbalances [29] particularly associated with D-amino acids [34] contributing to most forms of disease and genetic degradation due to not having whats required to perform highly level RNA binding it's clearly designed for [3]. In addition to defective genetic expression some forms of splicing are unable to complete processes such as hydrolytic splicing [19] previously discussed in the original abandoned draft. 5-meo-DMT was also recently scientifically confirmed as having a role in RNA gene expression in studies unable to identify the pathways and actions by which this was so [24].
To oversimplify such complex concept for most of the week Tryptophan takes care of the amino offspring in the beautiful house she built with DMT who's basically away most the time working the equivalent of FIFO. These two are like the perfect couple with such great chemistry that even after billions of years when they finally see one another the rules change as DMT and Tryptophan bend one another over in ways science could even begin to imagine between pulling the kids into line working together as a family to get on top of the fucken house. In an ideal world DMT's only been gone for a week so providing there's been no major issues after a quick once over they can invest their energy towards renovations to keep up with the joneses. However, whilst Trypophan's literally a goddess, without a decent services of good hard D regular enough she inevitably falls behind. One of the facts of life is although Tryptophan couldn't handle him there all the time there are certain things she simply cannot do right without DMT some of which are of critical significance requiring them both to work as a team to ensure the kids do their chores on time and precisely how they're fucken told.
According to PubChem and CHebi at a pH of 7.3 DMT in it's ammonium form N,N-Dimethyltryptaminium is classified as live "Major Mircospecies" and Tryptophan and other essential protonated amino acids in appropriate forms are live "Major Species" alluding to the possibility that they are individual entities, or perhaps a set of interconnected conscious equal opposite entities who worked quite well together for Four Billion years until humans severed thier loving relationship. High-level quantum mechanical computations, indicate that in gaseous state, as well as in the presence of single water molecule acting as a catalyst, and also in a water solvated environment - the reaction barrier is reduced making the aminium cation prone to inversion. Interestingly, in contrast to molecules with a carbon atom stereocenter, stereo-inversion with a nitrogen stereocenter was observed as a three multi-step process with quite high, or perhaps particular, reaction barrier. Although "gas-phase stereoinversion in the aminium cation is predicted to be infeasible, even along the proton transfer pathway which involves significant quantum tunneling" (Kaur, Ramanpreet & Vikas,. 2017) [18]. Looking back to learn this was independently identified prior to reciting in the comprehensive work of Marija Liutkute, et al (2020) [6] conveniently and reassuringly on this very same subject.
Consciousness over applying the above to the selection of placing any of the countless possible stereochemical combinations together as required is the only plausible explanation for the level of harmonious intermolecular coordination associated with the consumption of DMT as an essential activating such processes. Although yet to come across others with such a strong stance on acknowledging our oldest known living ancestors who played a critical role in consciously collectively contributing to the coevolution of all life on earth. In addition to their official classification there is no shortage of evidence supporting the fact that even in their basic ridged configuration amino acids have enough live in them to operative as heretical teams with influence over one another and a broad range of ecological contexts [6, 10, 11, 13, 14, 15, 16, 17, 18]. Considering this in contexts to N,N-Dimethlytryptaminium being a Major Microspecies associated with this group Major Species depending on language and cultural background some might even consider at the very least the "God" of the "Amino Acids". However, combining consciousness with the with scientific evidence of reactions to amino acids reflected across the coevolution of biodiversity of all living organisms across the globe their dominance is perhaps the most flawless examples of a natural coevolutionary synergetic equilibrium in the history of right up until humans came along and fucked everything up.
Before proceeding to explore the protonated amino acids the picture shows the N-terminal beside the "ancient RNA-binding motif" beneath crystal structures of two Sm dimers, namely, D1D2 and BD3, revealed that the Sm motif represents an autonomously folding domain consisting of an N-terminal helix followed by five -sheets (21). [2]
Considering plausible possibilities for the mysteriously elusive meaning of [trypt] in N,N-Dimethyl-[trypt]-amine which has proven impossible get a straight answers on, Tony suggested the appropriately fitting [Tryp] = Bore [T] = Period of oscillation?
Although having tentitively sat with that new information has revealed that ".Kühne named "trypsin" (a byproduct of Tryptophan) "from the Ancient Greek word 'thrýpto' which means 'I break' or 'I break apart'.[6]" [93]. Regardless, both are relevant due to trypanosoma sharing names and based of evidence suggesting they play synergetic active role in their host environments subject variable pH activating secondary amino acid configuration at around pH 7.3. However, "I break apart" prefixed by [T] = "Period T time of Oscillation" (when in the presence of DMT or Tprytophan) supports the secondary amino configurations. This is also relevant to Mast Cells (MC's) and their interlationship with all orans particularly bone marrow, the heart [94] and skin in addition to the cnetral nervous system beong the one organ in which they are not found [sigtations in previous interconnected work] . I personally healed a damaged corinary artery after a SCAD heart attack with Ayahausca releaving around 8 months of persistent chest pain whlist building up the courage to follow through with what I had every reason to believe would help me. Although trypanosoma are often associated with pathenogenic responses [96] this is likely due to problems associated with a lack of regular tryptamine with needs to be urgently reonsidered .as an essential intermittently required indolamine. Trypanosoma are also associated with nano tubes [95] which is the amino acid related subject that lead me back to the nomenclature of "TRYPT".
This will be covered in greater detail after examining the amino acids. However, the nature of this creepy set of "bore holes" to? Fits with the above account of Trypt, the difference between DMT and Tryptophan sexually being Major Microspecies and Species due to DMT's tiny cock and neurotransmitter Atlas indicating male "unknown neurotransmitter" [29].
Nevertheless, the product is likely [trypt]ase which is apparently "a household name for allergists and immunologists but a mysterious term for the rest of the medical specialties, who do not know its origin, use, and significance. Four human tryptases have been described: α, β, γ, and δ, two of which, α and β, are measurable in blood and other human body fluids. Tryptases are serine proteases and the most abundant protein components in mast cell secretory granules.1 Tryptases are found in all mast cell phenotypes, including connective tissue mast cells and mucosal mast cells,2 with small amounts detected in basophils, accounting for approximately 0.4% of that found in mast cells.3" [61]. This is of extreme value with two obvious testing units of measure potentially relevant to urine and serum samples associated with Universal Asylums experimental research yet otherwise not present without DMT, etc included in the amino configuration. Additionally there is also the gastrointestinal and respiratory tract significance to analysis of stool and saliva / respiratory excretion samples. I stumbled upon this as my experiments indicated that DMT is likely of benefit to anaphylaxis which which is overrepresented in “white people” who are likely to present greater levels of intergenerational genetic degradation subject to oppressive culture. Serine is in bold red for a reason which will become clear upon examination of it's definition in relation to the secondary configuration hypothesis.
In addition to Serine , Tyrosine's association with Mast Cells and mTOR [64] is also of great interest being the only amino labeled "the major species" in the appropriate form under the right conditions. The significance of both Serine and Tyrosine to the complexities of the proposed secondary amino configuration should be self explanatory upon examination of their definitions in context to that of associated “amines”. Particularly in relation to reverse configuration of such systems and the complex dynamic interrelationships with associated pathways and/or counterparts. The name "Mast Cells" is interesting too as [Mast] could well be representative of the mast of a ship which has a role in direction and prior to investigation is possibly an abbreviation of “master” although arguably only to the vessel which remains subject to it’s ecological context.
Hang on ...
Whilst levels of symbiosis vary, after Billions of years of coevolution and due to the very nature of life itself it's hard to argue that any speacies is or possibly even ever was completely independent. A recent study titles "A neo-functionalized homolog of host transmembrane protein controls localization of bacterial endosymbionts in the trypanosomatid Novymonas esmeraldas" confirms various variations in potien host coevolution. These include Strigomonas galati, a human tryptasomatid, featuring a preserved charged tail with the characteristic motif SKKXQ rather than a C terminus discribed as deleted, in addition to a number of variations in C and N protine terminius lengths [88]. Although, malaigned according to multiple sequence alignment software version 7 conserved proteins considered, "the loop between TMH2 and TMH3 in TMP18e is divergent and predicted to contain a single a-helical turn" [88]. Whilst distant in this sense Strigomonas galati likely fits one of the motifs in the Sm Domain it also shares similarities with fits with Herpetomonas muscarum and Angomonas deanei which along side the Sm Domains N terminus helix, and the terminuses of Lsm1 complex and Pat1 protein could possibly indicate they share human hosts. Genetically the former would be the equivelent of an aunt and the latter a sibling [88]. However, the motif suggests there are likely others too. However, it would seem likely that the aminos connect to the N terminus playing a biochemical role in unfolding the crystal structure enabling access to the motifs [91, 92] and also in providing additional support such as pt nanotubes, biochemical / biophysiological roles and neuro transmittion coverd in greater detail at some point in the following.

According to Gawryluk ( 2023) Trypanosomatida, a group of insect- vectored obligate parasites that includes numerous medically relevant species, including those in the genera Trypanosoma and Leishmania. And although Novymonas is a close relative of Leishmania, it is not a pathogen. Rather, it is an insect-limited parasite that inhabits the hind-gut of a bug, Niesthrea vincentii(Hemiptera: Rhopalidae), and was first isolated during a diversity survey in Ecuador. Novymonas harbours a small but variable number of b-proteobacterial symbionts, and alternates between a substratum-attached proliferative ‘rosette’ stage and an elongated motile ‘swimmer’ stage [89]. Whilst there are many questionable aspects of the article it provides further confirmation of evidence between the interrelationships between amino acids, tryptanosoma and even short nano-tubules which is the first evidence I have seen that's even close to what I'm more confidently and comprehensively trying to articulate. This raises the question of conciousness again and weather it's the aminos or the tryptanosoma and if so which one? or ones? Or do different ones share concioousness with different ions, etc. The greatest opssisite view is that a true symbiotic relationship between a host with countless commplex interrelationships is that feeding them DMT to awaken them, not only raises their health but doing so clears out the system.
Zakharova A, Saura et al (2021) "The closest relative of human pathogen Leishmania, the trypanosomatid Novymonas esmeraldas, harbors a bacterial endosymbiont "Candidatus Pandoraea novymonadis." Based on genomic data, we performed a detailed characterization of the metabolic interactions of both partners. While in many respects the metabolism of N. esmeraldas resembles that of other Leishmaniinae, the endosymbiont provides the trypanosomatid with heme, essential amino acids, purines, some coenzymes, and vitamins. In return, N. esmeraldas shares with the bacterium several nonessential amino acids and phospholipids. Moreover, it complements its carbohydrate metabolism and urea cycle with enzymes missing from the "Ca. Pandoraea novymonadis" genome. The removal of the endosymbiont from N. esmeraldas results in a significant reduction of the overall translation rate, reduced expression of genes involved in lipid metabolism and mitochondrial respiratory activity, and downregulation of several aminoacyl-tRNA synthetases, enzymes involved in the synthesis of some amino acids, as well as proteins associated with autophagy. At the same time, the genes responsible for protection against reactive oxygen species and DNA repair become significantly upregulated in the aposymbiotic strain of this trypanosomatid." [90]. The beginning of this abstract highlights the problematic nature of stating something to be pathagenic and continues to concicely map out the complexities of deleting any component that distorts the equalibrium creating additional problems for every attempted solution. Whilst starving them is one way feeding them is another. However, the only way to accurately study them is within a natural host. The fact that I am now 100% convinced [TRYP] = Tryptanosmatid suffixed by [T] = period of ossilation and that this has been known and hidden is frightening AF.
Due to the fact that mast cells are distributed acrss all aspects of our bodies asssides from the central nervous system I would expect the tryptanosomatida species that lives in them are our oldest most dominant natural symbiotic relationships and that the amino acids someway connect them to the centeral nearvous system. If mast is in fact short for “master” it is in fact possibly that from there the aminos play a role conecting them other cell groups hosting other tryptanosomatida species. For example whilst Strigomonas galati & potentially two other motif tailed species are equipped to connect to the Sm domain via the N terminal helix which also possibly acts as an anchor [92] for nanotubes, and perhaps in some way connects to the Lsm1 complex. However, the Lsm1 complex is also and perhaps exclusively able to connect to Tryptanosomatida species with C terminus tails such as Herpetomonas muscarum or Angomonas deanei which are potentially capable of connecting to TMP18 as the most universal of proteins [88]. There's also the possibility of certain species coeecting to the amino acid system an consuming certain protienes fueling the aminos throughout polipeptide production. Without a familiarity of 100s of combinations species of protine systems it is nessesary to speak in principle. Whilst this could apply to Pat1 protine which will be discussed at some point after taking a look at amino acids it is possibly that trypt-amines connects directly .to Pati1 although the former seem more likely. Regardless the complexity of such complex combinations symbiotic relationsihps presents major challanges for the latest combinations which the above studies are helping resolve.
Introducing what could well be our earliest known anscetors:
Tryptophan
Tryptophan (CHEBI:27897) is not live but is tautomer of...
Tryptophan Zwitterion (CHEBI:64554) obtained by transfer of a proton from the carboxy to the amino group of tryptophan is a Major Species at pH 7.3....
Incoming connection to both...
L-tryptophan zwitterion (CHEBI:57912) which is a Major Species at pH 7.3
D-tryptophan zwitterion (CHEBI:57719) having an anionic carboxy group and a protonated α-amino group a Major Species at pH7.3
D-Tryptophan is neither live nor a major species. However, is a carrier protiene and has a ligand protien structured with a highly useful extension so it's here for now encase of future relevance [13].
N,N-Dimethyltryptamine (CHEBI:28969) is not classed as live or a species but a conjugate base of...
N,N-Dimethyletryptanimium (CHEBI:193124 ) is a "LIVE" Major Mircrospecies at pH 7.3 [10].
Tryptaminium (CHEBI:57887) An ammonium ion that is the conjugate acid of tryptamine arising from protonation of the primary amino group; major species at pH 7.3.
Serotonin(1+) (CHEBI:350546) An ammonium ion that is the conjugate acid of tryptamine arising from protonation of the primary amino group; major species at pH 7.3.
The interesting this about serotonin(+1) and N,N-Dimethyletryptaminium is the aromatic position of their six member rings given DMT's methyle's can be used and exchanged in any combination to place any molecule anywhere it wants to spread any ring.
Furthermore, N,N-Dimethyletryptaminium is classed as "Major Microspecies" as opposed to "Major Species".
Perhaps we'll leave DMT unitil Last
As tryptophan has been covered what western medicine claims are the remaining Eight "essential" followed by the Eleven "non essential" standard and then the Three remaining protonated Amino Acids will be the following will summarise where possible otherwise directly quote PubChem and Chebi unless otherwise sighted;
Eight "essential" amino acids:
1) Phenylalanine
D-phenylalanine zwitterion is a D-alpha-amino acid zwitterion that is D-phenylalanine in which a proton has been transferred from the carboxy group to the amino group. It is the major species at pH 7.3
2). Valine
L-valine zwitterion is an L-alpha-amino acid zwitterion obtained by transfer of a proton from the carboxy to the amino group of L-valine; major species at pH 7.3.
3). Threonine
L-threonine zwitterion is zwitterionic form of L-threonine arising from transfer of a proton from the carboxy to the amino group; major species at pH 7.3.
4). Isoleucine
L-isoleucine zwitterion is an L-alpha-amino acid zwitterion obtained by transfer of a proton from the carboxy to the amino group of L-isoleucine; major species at pH 7.3
5). Methionine
L-methionine zwitterion is zwitterionic form of L-methionine having a anionic carboxy group and a cationic amino group; major species at pH 7.3.
6). Histidine
L-histidine is the L-enantiomer of the amino acid histidine. It has a role as a nutraceutical, a micronutrient, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a human metabolite, an algal metabolite and a mouse metabolite. It is a proteinogenic amino acid, a histidine and a L-alpha-amino acid. It is a conjugate base of a L-histidinium(1+). It is a conjugate acid of a L-histidinate(1-). It is an enantiomer of a D-histidine. It is a tautomer of a L-histidine zwitterion [which includes an anionic carboxy group and a protonated alpha-amino group as a polar amino acid zwitterion]... D-histidine is an optically active form of histidine having D-configuration. It has a role as a Saccharomyces cerevisiae metabolite. It is a D-alpha-amino acid and a histidine. It is a conjugate base of a D-histidinium(1+). It is a conjugate acid of a D-histidinate(1-). It is an enantiomer of a L-histidine. It is a tautomer of a D-histidine zwitterion [a polar amino acid zwitterion restulting from the transfer of a proton from the carboxy group to the alpha-amino group & The major species at pH 7.3]...*All of which asside from the zwitterions appear to include every combination of (+1 & +2) & (-1 & -2) variations...
7). Leucine
D-leucine zwitterion is a D-alpha-amino acid zwitterion arising from the transfer of a proton from the carboxy to the amino group of D-leucine; major species at pH 7.3.
8). Lysine
N(6)-acetyl-L-lysine zwitterion is an amino acid zwitterion obtained via transfer of a proton from the carboxy to the amino group of N(6)-acetyl-L-lysine; major species at pH 7.3.
According to PubChem (2024) "L-lysine is an L-alpha-amino acid; the L-isomer of lysine. It has a role as a micronutrient, a nutraceutical, an anticonvulsant, an Escherichia coli metabolite, a Saccharomyces cerevisiae metabolite, a plant metabolite, a human metabolite, an algal metabolite and a mouse metabolite. It is an aspartate family amino acid, a proteinogenic amino acid, a lysine and a L-alpha-amino acid. It is a conjugate base of a L-lysinium(1+). It is a conjugate acid of a L-lysinate. It is an enantiomer of a D-lysine. It is a tautomer of a L-lysine zwitterion and a L-Lysine zwitterion." [32] "Lysine (abbreviated as Lys or K) is an α-amino acid with the chemical formula HO2CCH(NH2)(CH2)4NH2. This amino acid is an essential amino acid, which means that humans cannot synthesize it. Its codons are AAA and AAG. Lysine is a base, as are arginine and histidine. The ε-amino group acts as a site for hydrogen binding and a general base in catalysis. Common posttranslational modifications include methylation of the ε-amino group, giving methyl-, dimethyl-, and trimethyllysine. The latter occurs in calmodulin. Other posttranslational modifications include acetylation. Collagen contains hydroxylysine which is derived from lysine by lysyl hydroxylase. O-Glycosylation of lysine residues in the endoplasmic reticulum or Golgi apparatus is used to mark certain proteins for secretion from the cell." [32].
In 2009 proteinaceous N-modification in lysine was discovered suggesting that the inclusion of DMT is likely to result in it acting as an animated amino group donor after which it is considered deaminated, which is reversible without distinction between acting as a donor or acceptor between direction as required subject to enzymic signals from DMT and possibly Tryptophan [30, 31]. Obviously this would not only allow the reverse of its actions but the reversibility of the above which is also likely to include countless possible combinations capable of serving a broad range of purposes far beyond current scientific understandings. Nε-methylation of lysine residues found on unstructured N-terminal histone tails and core histones indicates a role in the regulation of of dynamic molecular scale amino interactions requiring continuous conscious complex calculations throughout time across countless combinations of variables associated with coevolving systems of life in their entirely [33].
Eleven "non essential" Amino Acids:
1). Alanine
L-alanine is the L-enantiomer of alanine. It has a role as an EC 4.3.1.15 (diaminopropionate ammonia-lyase) inhibitor and a fundamental metabolite. It is a pyruvate family amino acid, a proteinogenic amino acid, a L-alpha-amino acid and an alanine. It is a conjugate base of a L-alaninium. It is a conjugate acid of a L-alaninate. It is an enantiomer of a D-alanine. It is a tautomer of a L-alanine zwitterion [ arising from transfer of a proton from the carboxy to the amino group; major species at pH 7.3]
Standard
2). Arginine
Arginine (CHEBI:29016) is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidine group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the l-arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid. The one-letter symbol R was assigned to arginine for its phonetic similarity.
3). Asparagine
asparagine (CHEBI:22653) (symbol Asn or N) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain carboxamide, classifying it as a polar (at physiological pH), aliphatic amino acid. It is non-essential in humans, meaning the body can synthesize it. It is encoded by the codons AAU and AAC. The one-letter symbol N for asparagine was assigned arbitrarily, with the proposed mnemonic asparagiNe; [Zwitterionic form of L-asparagine arising from transfer of a proton from the carboxy to the amino group; major species at pH 7.3.]
4). Aspartic acid
Aspartic acid (CHEBI:22660) An α-amino acid that consists of succinic acid bearing a single α-amino substituent. Capable of Hydrogen donor or acceptor.
Aspartic Acid has an overall negative charge and plays an important role in the synthesis of other amino acids and in the citric acid and urea cycles. Asparagine, arginine, lysine, methionine, isoleucine, and some nucleotides
5). Cysteine
"L-cysteine is an optically active form of cysteine having L-configuration. It has a role as a flour treatment agent, a human metabolite and an EC 4.3.1.3 (histidine ammonia-lyase) inhibitor. It is a serine family amino acid, a proteinogenic amino acid, a cysteine and a L-alpha-amino acid. It is a conjugate base of a L-cysteinium. It is a conjugate acid of a L-cysteinate(1-). It is an enantiomer of a D-cysteine. It is a tautomer of a L-cysteine zwitterion."... "Cysteine is a uremic toxin. Uremic toxins can be subdivided into three major groups based upon their chemical and physical characteristics: 1) small, water-soluble, non-protein-bound compounds, such as urea; 2) small, lipid-soluble and/or protein-bound compounds, such as the phenols and 3) larger so-called middle-molecules, such as beta2-microglobulin. Chronic exposure of uremic toxins can lead to a number of conditions including renal damage, chronic kidney disease and cardiovascular disease. Cysteine is a naturally occurring, sulfur-containing amino acid that is found in most proteins, although only in small quantities. Cysteine is unique amongst the twenty natural amino acids as it contains a thiol group. Thiol groups can undergo oxidation/reduction (redox) reactions; when cysteine is oxidized it can form cystine, which is two cysteine residues joined by a disulfide bond. This reaction is reversible: as reduction of this disulphide bond regenerates two cysteine molecules. The disulphide bonds of cystine are crucial to defining the structures of many proteins. Cysteine is often involved in electron-transfer reactions, and help the enzyme catalyze its reaction. Cysteine is also part of the antioxidant glutathione. N-acetyl-L-cysteine (NAC) is a form of cysteine where an acetyl group is attached to cysteine's nitrogen atom and is sold as a dietary supplement. Cysteine is named after cystine, which comes from the Greek word kustis meaning bladder - cystine was first isolated from kidney stones. As cysteine contains a sulphydryl group, it can undergo redox reactions. Oxidation of cysteine can produce a disulfide bond with another thiol, or further oxidation can produce sulphfinic or sulfonic acids. The cysteine thiol group is also a nucleophile and can undergo addition and substitution reactions. Thiol groups become much more reactive when they are ionized, and cysteine residues in proteins have pKa values close to neutrality, so are often in their reactive thiolate form in the cell. The thiol group also has a high affinity for heavy metals and proteins containing cysteine will bind metals such as mercury, lead and cadmiumtightly. Due to this ability to undergo redox reactions, cysteine has antioxidant properties. Cysteine is an important source of sulfur in human metabolism, and although it is classified as a non-essential amino acid, cysteine may be essential for infants, the elderly, and individuals with certain metabolic disease or who suffer from malabsorption syndromes. Cysteine may at some point be recognized as an essential or conditionally essential amino acid. Cysteine is important in energy metabolism. As cystine, it is a structural component of many tissues and hormones. Cysteine has clinical uses ranging from baldness to psoriasis to preventing smoker's hack. In some cases, oral cysteine therapy has proved excellent for treatment of asthmatics, enabling them to stop theophylline and other medications. Cysteine also enhances the effect of topically applied silver, tin and zinc salts in preventing dental cavities. In the future, cysteine may play a role in the treatment of cobalt toxicity, diabetes, psychosis, cancer and seizures."
6). Glutamic Acid
L-glutamic acid is an optically active form of glutamic acid having L-configuration. It has a role as a nutraceutical, a micronutrient, an Escherichia coli metabolite, a mouse metabolite, a ferroptosis inducer and a neurotransmitter. It is a glutamine family amino acid, a proteinogenic amino acid, a glutamic acid and a L-alpha-amino acid. It is a conjugate acid of a L-glutamate(1-). It is an enantiomer of a D-glutamic acid.
7). Glutamine
L-glutamine zwitterion is an amino acid zwitterion arising from transfer of a proton from the carboxy to the amino group of L-glutamine; major species at pH 7.3. It has a role as a metabolite. It is an amino acid zwitterion and a polar amino acid zwitterion. It is a tautomer of a L-glutamine.
But wait there's more
And more...
8). Glycine
Glycine is the simplest (and the only achiral) proteinogenic amino acid, with a hydrogen atom as its side chain. It has a role as a nutraceutical, a hepatoprotective agent, an EC 2.1.2.1 (glycine hydroxymethyltransferase) inhibitor, a NMDA receptor agonist, a micronutrient, a fundamental metabolite and a neurotransmitter. It is an alpha-amino acid, a serine family amino acid and a proteinogenic amino acid. It is a conjugate base of a glycinium. It is a conjugate acid of a glycinate. It is a tautomer of a glycine zwitterion.[an amino acid zwitterion arising from transfer of a proton from the carboxy to the amino group. It is also a fast inhibitory neurotransmitter.]
9). Proline
L-proline is pyrrolidine in which the pro-S hydrogen at position 2 is substituted by a carboxylic acid group. L-Proline is the only one of the twenty DNA-encoded amino acids which has a secondary amino group alpha to the carboxyl group. It is an essential component of collagen and is important for proper functioning of joints and tendons. It also helps maintain and strengthen heart muscles. It has a role as a micronutrient, a nutraceutical, an algal metabolite, a Saccharomyces cerevisiae metabolite, an Escherichia coli metabolite, a mouse metabolite and a member of compatible osmolytes. It is a glutamine family amino acid, a proteinogenic amino acid, a proline and a L-alpha-amino acid. It is a conjugate base of a L-prolinium. It is a conjugate acid of a L-prolinate. It is an enantiomer of a D-proline. It is a tautomer of a [L-proline zwitterion: the predominant species at physiological pH]
10). Serine
L-serine (CHEBI:17115) Serine (symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+3 form under biological conditions), a carboxyl group (which is in the deprotonated −COO− form under biological conditions), and a side chain consisting of a hydroxymethyl group, classifying it as a polar amino acid. It can be synthesized in the human body under normal physiological circumstances, making it a nonessential amino acid. It is encoded by the codons UCU, UCC, UCA, UCG, AGU and AGC.
11). Tyrosine
L-tyrosine is an optically active form of tyrosine having L-configuration. It has a role as an EC 1.3.1.43 (arogenate dehydrogenase) inhibitor, a nutraceutical, a micronutrient and a fundamental metabolite. It is an erythrose 4-phosphate/phosphoenolpyruvate family amino acid, a proteinogenic amino acid, a tyrosine and a L-alpha-amino acid. It is functionally related to a L-tyrosinal. It is a conjugate base of a L-tyrosinium. It is a conjugate acid of a L-tyrosinate(1-). It is an enantiomer of a D-tyrosine. It is a tautomer of a L-tyrosine zwitterion.
D-tyrosine zwitterion is a D-alpha-amino acid zwitterion that is D-tyrosine in which a proton has been transferred from the carboxy group to the amino group. It is the major species at pH 7.3.
New Two
Selenocysteine
Selenocysteine (CHEBI:9093) An α-amino acid that consists of alanine where one of the methyl hydrogens is substituted with a seleno group. Capable of donating or accepting hydrogen.
Pyrrolysine
pyrrolysine (CHEBI:91273) An N-acyl-amino acid that is lysine in which one of the amino nitrogens at position N6 is replaced by a 3-methyl-3,4-dihydro-2H-pyrrole-2-carbonyl group.
Lucky Last
N-formylmethionine
N-formyl-L-methionine is a L-methionine derivative in which one of the hydrogens attached to the nitrogen is replaced by a formyl group. It has a role as a metabolite. It is a proteinogenic amino acid, a N-formyl amino acid and a L-methionine derivative
N-Formylmethionine is effective in the initiation of protein synthesis. The initiating methionine residue enters the ribosome as N-formylmethionyl tRNA. This process occurs in Escherichia coli and other bacteria as well as in the mitochondria of eucaryotic cells.
N-formyl-L-methioninate (CHEBI:57809) conjugate base of N-formyl-L-methionine; major species at pH 7.3.
Oh... DMT!
N,N-dimethyltryptaminium Molecular Formula (MF) C12H17N2+ (DMT+) described as a major microspecies at pH 7.3 it is a conjugate acid of a N,N-Dimethyltryptamine MF C12H16N2 (DMT) With a Hydrogen Bond Donor Count of 2. DMT and DMT+ also have only one Covalently-Bonded Unit Count which is presumably H2O (Or is it?). DMT and DMT+ what are also highly "Canonicalized".
DMT(+) have a rotatable bond count of three which I am unsure I understand at this point in time (Wicker & Cooper, 2016) or how it fits in relation to two Methyl groups attached to an N / NH+ Amine/Aminium Nitrogen Atom.
One of these includes CH3 (Methyl Radical) which is basically a Carbon (C) Atom linked central to Three Hydrogen (H) Atoms. CH3 is organic and Canonicalised with a "Mixtures, Components, and Neutralized Forms (MCNF) Count: of 53". This doesn't seem like much until digging a little deeper and finding Methyl Radiacl Ch3 cooccurences with H^ (Hydrogen) , C^^^^ (Carbon) and CH3+ (Methylium). Methylium has a MCNF Count: of 1767 both followed by way too much awesome shit to list (PubChem, 2024).
H3C has been a little trickier. Initially the closest match I could get serching H3C via PubChem was the gene "N-(2,4-dimethylphenyl)-2,2,2-trifluoro-N-[(E)-(3-methoxyphenyl)methylideneamino]acetamide" which "coincidentally?" performs countless fitting actions (PubChem, 2024). Although noteworthy should it later prove to be significantly associated, I tried breaking H3C down into individual elements in the following serches.
According to PubChem H on it's own comes up with one result H(.) (Hydrogen) which is canocalized and has a Formal Charge (FC) of 0. However, searching H3 comes up with 3 results H3+3 (FC: 3), H3+ (FC 1) and H3-3 (FC -3) all canocalized. Whilst C only comes up with one result C^^^^ (Carbon) which is canocalized and includes the following 25 Chemical Co-Occurrences in Literature: Nitrogen, Water, Oxygen, Hydrogen, D-Glucose, Sulfur, Phosphorus, Carbon Dioxide, Sucrose, Iron, Methane, Lithium, Nitrate, Acetate, Silicon, Silicon Dioxide, Gold, Butyric Acid, Copper, Ethanol, Phosphate Ion, Methanol, Hydroxyl radical, Platinum, Sodium Chloride. Carbon also, interestingly has a Gene Count of 5395 and 100 Chemical- Gene Co-Occurrences in Literature.
According to Warren (2014) a methyl group consists of three hydrogen atoms surrounding one carbon atom, symbolized as CH3 and are capable of attaching directly to DNA by forming a chemical bond with cytosine to form 5-methylcytosine.
Nian-Dong (2021) advises that Five- and Six-membered aromatic rings bearing a methyl group are important molecular fragments, as the methyl group can play a pivotal role in improving the biological activities due to its capability in modulating the molecular conformation as well as the physical and chemical properties.
1956 “It has been found that N,N-dimethyltryptamine oxide will undergo a ferric ion induced rearrangement in aqueous solution to give N-methyltryptamine and formaldehyde or formic acid. The reaction, which provides a model for biological N-dealkylation, was studied under a variety of conditions.” [82].
Ferric cation (ion): "Molecular Formula Fe+3Iron(3+) is an iron cation and a monoatomic trication. It has a role as a human metabolite, an Escherichia coli metabolite, a mouse metabolite and a cofactor."
"ChEBI
Iron is a transition metal with a symbol Fe and atomic number 26. By mass, it is the most common element
on Earth. Iron is an essential element involved in various metabolic processes,including oxygen transport, deoxyribonucleic acid (DNA) synthesis, and energy production in electron transport. Resulting from inadequate supply of iron to cells due to depletion of stores, iron deficiency is the most common nutritional deficiency worldwide, particularly affecting children, women of childbearing age, and pregnant women. Iron deficiency may be characterized without the development of anemia, and may result in functional impairments affecting cognitive development and immunity mechanisms, as well as infant or maternal mortality if it occurs during pregnancy. The main therapeutic preparationofironis[DB13257],andiron-sucrosemayalsobegivenintravenously. Ironexistsintwo oxidation states: the ferrous cation (Fe2+) and ferric cation (Fe3+). Non-haem iron in food is mainly in the ferric state, which is the insoluble form of iron , and must be reduced to the ferrous cation for absorption. Ferric citrate (tetraferric tricitrate decahydrate) is a phosphate binder indicated for the control of serum phosphorus levels in patients with chronic kidney disease on dialysis" .[83].

"It is proposed that there may be separate cellular uptake pathways for ferrous iron and ferric iron . While ferrous iron is primarily carried by divalent metal transporter-1 ( DMAT -1) [pictured to the left], cellular uptake of ferric iron is predominantly mediated by beta-3 integrin and mobilferrin,
which is also referred to as calreticulin in some sources as a homologue. However, the most dominant pathway in humans is unclear" [83, 84]. Subject to ones definition of "unclear" and understanding of DMT.
“8.1 Pharmacodynamics
When Fe3+ is converted to soluble Fe2+, it primarily exists in the circulation in the complex forms bound to protein (hemoprotein) as heme compounds (hemoglobin or myoglobin), heme enzymes, or nonheme compounds (flavin-iron enzymes, transferring, and ferritin). Once converted, Fe2+ serves to support various biological functions. Iron promotes the synthesis of oxygen transport proteins such as myoglobin and hemoglobin, and the formation of heme enzymes and other iron-containing enzymes involved in electron transfer and redox reactions. It also acts as a cofactor in many non-heme enzymes including hydroxylases and ribonucleotide reductase. Iron-containing proteins are responsible in mediating antioxidant actions, energy metabolism, oxygen sensing actions, and DNA replication and repair. Saturation of transferrin from high concentrations of unstable iron preparations may elevate the levels of weakly transferrin-bound Fe3+, which may induce oxidative stress by catalyzing lipid peroxidation and reactive oxygen species formation.”
Metal transportation is obviously essential for all forms of life [85] with various compartmentalised systems which remain intact after billions of years of coevolution competing with algae for nutrients & to expel waste since life began under the ocean. At this stage it is extremely difficult to acquire information regarding the iron ion of DMT which evidence suggests plays a dominant role in metal transportation.

Finishing up with DMT and β-Carboline and exploring various pathways will lead into discussion of the significance Platinumnanotubes which I suspected likely linked to a range of more complex multifunctional transitional metal structures such as the fullerene complexes along the lines of [[Ph3P]2Pt]6(η2-C60) illustrated to the left which Balch and winkler (2021) cover in far greater detail [86]. Whilst much could be said about these in relation to facilitating the following processes whilst linking networks of transitional metal nanotubes and C C bonded structural arrangements for the sake of simplicity it is best to keep the depiction in mind whilst considering the following.
Additionally as Universal Asylums experimental research involved DMT in the form of Ayahausca including variations of β-Carboline from Syrian Rue and B. Caapi, in addition to a set of experiments involving Mesculine the following chart and it's direct quotation from it's source [45] have been included.
Fig1. Molecular structures of N,N-dimethlytryptamine (DMT) and other psychedelics, the main ayahuasca β-carbolines, and key metabolic pathways.
A Indole and benzene rings (gray) are the chemical scaffolds of the two main categories of psychedelics, i.e. tryptamines (red) and phenethylamines (yellow). Serotonin, DMT and 5-MeO-DMT are structurally similar; LSD, while also containing the tryptamine (and phenethylamine) motif, is an ergoline derivative. Among the phenethylamine psychedelics, we present the structures of 4-bromo-2,5-dimethoxyphenethylamine (2C-B) and mescaline [45]. Universal Asylums experimental research includes syrum samples where it was not possible to precipitate suboxone withdrawal in a Human test subject via the administrations of naloxone whilst under the influence of mescaline.
B The β-carboline scaffold of harmine, harmaline, and tetrahydroharmine (THH) are shown in blue. [45].
C) These main β-carbolines in ayahuasca undergo demethylation to harmol, tetrahydroharmalol, and harmalol, respectively. Several cytochrome (CYP) enzymes are implicated in the demethylation of harmine and harmaline, but details are lacking for THH. Harmine and harmaline can also undergo ring-hydroxylation catalyzed by CYP450 [107, 108]. An additional metabolic route of harmaline is its oxidation to harmine. DMT is predominantly metabolized by oxidative deamination via monoamine oxidase type A (MAO-A), followed by formation of indole-3-acetic acid (3-IAA) by non-specific aldehyde dehydrogenases. Alternately, DMT is oxidised to DMT-N-oxide (DMT-NO) by CYP450 or demethylated by CYP2D6 and CYP2C19 to N-methyltryptamine (NMT), or hydroxylated to 6-hydroxy-DMT by yet unknown enzymes [60, 107, 109, 110]. Red arrows indicate inhibition of DMT metabolism by the β-carboline MAO-A inhibitors, resulting in lesser formation of 3-IAA [45].
Interesting that details are lacking for tetrahydroharmine given the position of it's aromatics which I believe would almost complete circuitry of the three way presumably dynamic connection with DMT, Tryptophan & Serotonin(1+) with it's odd aromatic position, via opening the five member ring β-carboline ring along with the five member rings of appropriate variations of of each of the others. This in conjunction with descriptions of the 14-16 membered ring systems mentioned earlier [40] along side the structure of the Peptide Transverse Centre [47] and in conjunction with DMT are of great foreseeable significance to the many unanswered questions regarding the ribosome [47]. In principle these fit with earlier discussion of the AMPARs including both a reversible mirror system in addition to comprehensive options for dynamic duel configurations. For example of the of countless additional dynamic combinations the following depiction is sighted from [47]. Theoretically this should also be able to run complex triple configurations using combinations of A, P and E channels.
In contrast to and/or perhaps complimenting "A neurotransmitter atlas of C. elegans males and hermaphrodites" (2024) [46] suggesting an unknown neurotransmitter of male "dominance" based on the worms: The previously quoted publication highlights that the authors of a particular studying into DMT and B, Caapi reported retraction of dendritic spines in the prefrontal cortex, but only in female DMT-treated rats [46]. Without exploring in detail, there is likely great value in contransting the variation between the C. Elegans worms, male and female rats and Universal Asylums proposal for a global study into snakes contrasting pythons and boas against others snakes and across geographic's outlined in Inclusion Body Disease Symptoms in Python "Cured" with "Ayahausca" after 40years of Science Blaming RNA Viruses: What Snakes can Teach Science About Evolution Before the UN & WHO Bioterrorist Attack!
An interesting question relating to the above is the fact that whilst DMT may inhibit various metabolic pathways whilst activating others in certain typical ways this has the potential to become extremely challenging for repeatable scientific testing due to it also having the potential to (dis)activate complex combinations of systems for different reasons across the system throughout time. However, Universal Asylums Opiod/Naloxon model offers a relatively objective solution to this, or at least about as close as it gets at this point in time. This also has it's limits and raises concerns for the "invention" in the Patent which I believe is a long acting opioid variation with the potential to provide useful data but also distort actions perhaps best observed under a range of conditions before getting too cocky about understanding them. Having said this Universal Asylum see countless technological opportunities associated with this such as covered in Not on my watch: Smart medical intervention & centralised medical grade data collection! which potentially can be linked into safety /protective clothing capable of administering localised DMT, etc in case of emergency requiring efficient management of trauma, and potential isolation or prioritisation of specific areas.
Now where were we?... That's right the fucken "Ancient RNA-Binding Motif", Sm Domain, Sml1 complex & PAT1. Where I started before finding a buch of better shit like usual!!
In their standard configuration amino acids are arguably live in a strict rigid kind of fashion in the sense of showing signs of hierarchy and influence between themselves and broader influence in serving their many objectives. At a pH of 7.3 DMT is capable of converting into what is scientifically classified as a live Major Mircrospecies along side a number of amino acid variations being live Major Species which will be identified shortly. Amino acids in their live actively charged states evidence suggest such entities collectively operate primarily between the N-terminal beside the "ancient RNA-binding motif" beneath crystal structures of two Sm dimers, namely, D1D2 and BD3, revealed that the Sm motif represents an autonomously folding domain consisting of an N-terminal helix followed by five -sheets (21). In both crystal structures, dimerization is brought about by an interaction of the 4 sheet of one Sm protein with 5 of the other, in line with biochemical and genetic evidence that the Sm domain is necessary and sufficient for the Sm proteins to form heteromers. Extrapolation of the Sm BD3 and Sm D1D2 structures, in conjunction with known interactions between the other Sm proteins, allowed the modeling of the Sm proteins into a sevenmember ring [2] and the mysterious unusually placed C terminal extension covering the opening of the Lsm1 complex which has had scientists baffled for years [3, 4, 5]. Essentially DMT(s) temporarily reconfigures the entire extracellular matrix by converting amino acids such as Tryptophan in live Major Species capable of working collectively at an intermolecular stereochemical level to from a distant perspective manage the entire extracellular matrix to facilitate thorough diagnostics and maintenance, including hardcore RNA genetic splicing in either direction. In addition to general maintenance and expression of 'tailor made' nutrition critical to maintaining levels of developmental divergence conducive of healthy coevolution. Regardless of rings which I believe I’ve now found up to 16 or so, there appears to be at least three Trypanosomatida species based on the motifs.
PAT1 Protein?
PAT1 is a unique protein that contributes to the RNA binding activity which include the distinguishing feature of N Terminal on one end and C Terminal rather than typical motifs of other protein groups and science is unable to explain why one half appears consistently remaining unused. Descriptions of used Pat1 protein consistently indicate that the remaining side that contains yeast and other properties that never get used creating all kinds of dis-regulation and degeneration which has science either rightfully concerned [6, 8]. Sometime scientists have even suggested PAT1 is a problem. However, Pati's job is to remain on standby for Tryptopan, Tryptamine and L-Tryptophan to arrive and remove the crystal structure covering the "ancient RNA-binding motif" and N Terminal so it can support processes associated with gene splicing and possibly other activity. When placed together puts them in a jar or otherwise inappropriate context about all the can do standing around like a bunch of council workers [ 6, 7, 8]. Therefore, Pati is NOT "responsible for translational repression and decapping activation, ultimately leading to mRNP degradation."[6]. It is entirely possible that "human Pat1b (PatL1) proteins also have conserved roles in the 5'→3' mRNA decay pathway" [6] However, this is most likely due to DMT deprivation.. Available evidence suggests that the C and N terminals complete circuitry to reconfigure systems and processes support RNA gene splicing between directions, and additional actions potentially requiring the otherwise unused Pat1 substrate that otherwise has little purpose but to remain on standby [7]. This may not be quit so simple though. Studies indicate the the N Terminal isn't required for yeast protein as opposed to the C-terminal extension which is required for RNA binding in vitro and Lsm1 function in vivo [22]. However, whilst it is likely that DMT in it's live Major Microspecies utilises the yeast protein portion of the PAT1 substrate along with glutamate and consumables associated with phosphorus, the reversibility of their fully activated state the N-terminal has the potential to come into play in otherwise unforeseeable manners.
According to Clair, A., et al (2019) “reverse splicing” is an essential activity that allows group II introns which are considered to be extremely old, likely first evolving in bacteria with other ribozymes in the primordial “RNA world - to colonize new genomic DNA locations through retrotransposition. Comparing the branched splicing pathway and hydrolytic splicing pathways performed by group II introns and the spliceome outlined in Fig. 1 of the article - the process of hydrolytic splicing which is described as linear is incomple. Quote Fig. 1. Splicing pathways performed by group II introns and the spliceosome. A) Branched splicing pathway. The bulged adenosine nucleotide attacks the 5′ SS in the first step, resulting a free 5′ exon and a lariat-3′ exon intermediate. The 3′-OH of the free 5′ exon attacks the 3′ SS in the second step to yield ligated exons and lariat intron. B) Hydrolytic splicing pathway. The 5′ SS is attacked by a water molecule in the first step, resulting in a free 5′ exon. The second step proceeds as in the branched splicing pathway to yield ligated exons and linear intron. [74].

Figure 1 (Clair A, et al., 2019) [74].
The first two steps are identical as branced splicing though the biopysiological catalysts involved in the first and third steps of branced spliceing depicted in A) aren't all together clear. With hydrolytic splicing (without DMT/DMT+ the first step involves a water molecule or hydroxide ion attacking and freeing the 5′ exon, the free 5′ exon subsequently appears to have an attempted hydroxide to attack the 3′ exon splice site which fails to lariat the free 3' intron. I'm new to biophysiological equasions or more so the new language which appears very deliberately set up to prevent science from firureing this one out. In my head presumably having used one hydrogen and one oxygen molecule in the hydroxide attack to achieve the first step, the second step would be more appropriately marked H (rather than HO) for the single remaining Hyrogen molecule which might be attracted to the 3′ exon splice site but unable to do much more than jizz in its face.
The crystalline structure built over the ancient motifs and N-terminal require precisely coordinated intermolecular stereochemical processes enabling the major species amino acids to break the rules of their standard configuration. There is no shortage of evidence of evidence of amino acids forming hierarchy and regulating conditions even on a nanoparticle scale [10, 13] which will receive further attention shortly. Stereo-inversion in aminiums is considered highly unlikely because of a high reaction barrier. However, this also provides stability in preforming the type of high level molecular activity involved in RNA splicing which have been calculated to be of extreme difference between 5 and 6 ring configurations [5]. With the potential to be far greater with mention of possible a 7 ring configuration above [2]. Consciousness combined with fully charged combinations of the Major Species amino acids in a hydrogen saturated environment fits with their coevolution of the Sm Domain of multicellular organism such as a human beings and other animals is arguably the only way this is likely to occur.
The substantial scientific evidence of Amino Acids having significant influence over transitional metals such as (Pt) Platinum and (Y) Yttrium, and substrates associates with conditions of different scientific research is of potential relevance on many levels [11, 12, 13]. Nanotubes caused and influenced by amino acids is a topic of great interest. The ≈8 nm diameter of platinum nanotubes [20] and the ≈8 nm diameter of the structures in B & C of Figure 1 [2] is of potential significance. In addition to potential actions in connecting the sight itself a plausible functional component of this could serve as an explanation for the phenomenon efficient means of purging processes associated with Ayahausca. One of these I have previously speculated in 2022 in Blocking Opiate & Benzodiazapine Withdrawal, & Entheogenic Purging of Toxins - via the Apendix? is the activation of the appendix expelling toxins into the lower gastrointestinal tract which is consistent with anecdotal evidence of people experiencing the type of sensations one might expect to be associated with that shortly prior to the sudden urge to defecate. Whilst of great science interest to medical science the problem often arises as to how to control them [11] which obviously is the live microspecies of the amino acids group offer a perfectly natural solution. Platinum is also considered to include 'species' with significant variation [21] yet to be fully explored in terms of the nature and definition of that. However, due to its prevalence in nature and interactions amino acids is also the most likely plausible explanation to the biophysicist description of the "ancient RNA-Binding motifs" having not real anchorage points and based on the appearance of the image [2] a such nanotubes would seem the only plausible explanation. Regardless they offer a useful evidence of the influence of amines which I'll hand over leading into some rather complex closing considerations regarding the subject.
To burst my bubble weeks since having identified the significance of Pt & Y nannotubes and their interrelationship with amino acids covered more in additional qoutations, “unrelated” enquiries have revealed that microtubules have been studied extensively. These have some distinct differences in C, Elegans consistent with an unknown neurotranmittor of male origin. They are also associated with lysine, tyrosine and other particularly relevant aminos [68, 69]. Essesnially, Universal Asylum is proposing that networks of Pt/Y nanotubes are the secondary configurations hardcore equivelent to microtubles and facilitate essential maintenance actions. However, before going into that I’ll leave the original contextual qoutes I haven’t got around to dissecting which may not be nessessary. .
The following quotes on the subject from an extensive study are of great interest supporting the above which create addition to complications or considerations for future scientific research in this area. "Lanreotide, a small peptide of eight amino acids, possesses all the molecular parameters driving its self-assembly in the presence of water. The self-assembly process of lanreotide is controlled by the balance between two opposite forces, i.e., hydrophobic effects, which result in water in an ‘‘attraction’’ between peptides and repulsive electrostatic interactions. Furthermore, the molecular parameters drive the molecular packing; and to obtain detailed information on these molecular parameters we adopted a site-directed mutational approach. We chose either to mutate residues that modify the conformation of the molecule or to successively substitute the three aromatic residues by a phenylalanine. Besides this precise purpose, the mutations we chose to apply to lanreotide are also related to more general questions. The influence of peptide conformation on molecular packing in self-assembly is, for example, relevant to amyloid peptides but also to bio- nanomaterials. In addition, p-stacking has been postulated as an important type of interaction driving amyloid fibril growth (3,27,55). Lanreotide and its derivatives are powerful tools in elucidating the involvement of Δ interactions and the peptide backbone conformation in peptide self-assembling processes. Moreover, understanding these types of interactions can help to design new molecules capable of forming desired supramolecular architectures." [13]. The above is of relevance due to their co-occurrence in plants undoubtedly consumed to an unknown extent by animals and humans throughout history likely to be included in ancient coevolutionary interrelationships within and between plants and animals. However, arguably should be understood before science gets to carried away creating new molecules.
"Which molecular parameters drive self-assembly?
The idea that Δ stacking is one of the driving forces of peptide-protein fiber formation emerged a few years ago. E. Gazit (56) reviewed studies on peptides extracted from the sequence of amyloid-forming proteins that kept the self- assembling properties of the entire protein. All these amino acid sequences contained a high number of aromatic residues. Knowing that aromatic residues are among the less frequently occurring amino acids, this observation is not trivial. Moreover, directed mutations systematically replacing these amino acids by Ala showed that all but the aromatic amino acids can be replaced by Ala without changing the propensity for self-assembly. Indeed, the replacement of the aromatic residues by Ala completely inhibited this capacity. It was further suggested that pp stacking provides an energetic contribution as well as order and directionality to the self-assembly of amyloid structures" [13]. This highlights the importance of aromatic amines such as DMT
The lanreotide sequence contains three aromatic amino acids in a total of eight. Besides these hydrophobic aromatic residues, it has two positive charges, which explain the high solubility of the peptide since nanotube assembly occurs at concentrations higher than 30 mM. Among the residues substituted in this study, we successively mutated the three aromatic residues into phenylalanine. We chose to mutate these aromatic side chains to another aromatic one and not, as Gazit did (56), to a simple Ala for two major reasons: i), we already knew from the segregation of the aromatic side chains in the self-assemblies that these residues played a major role involving Δ stacking, and ii), we wanted to know if these aromatic residues had some specific features. The minimal mutations DNal-DPhe and Tyr-Phe strongly affect the solubility of the peptide. Indeed, these two mutants do not form nanotubes or any other nanoscale self-assemblies based on extended intermolecular b-sheet, at least in the concentration range studied in this work, i.e., concentrations up to 10 times the critical concentration of lanreotide (300 mM). These results indicate that these mutations together increased the solubility of the peptide and decreased its self-assembling propensity. The aromatic interactions in lanreotide nanotubes, consequently, not only are related to simple pp interactions but also involve some more specific interactions. For information, we also tested the mutation of DNal into DAla. The behavior of this peptide was comparable to that of the mutant reported in this work (data not shown). From this study, we conclude that two of the three aromatic residues in the lanreotide sequence are directly involved in the intermolecular peptide-peptide interaction. These results are also in agreement with the idea developed by C. Dobson that to avoid protein fiber formation, nature has developed strategies to increase the solubility of the protein in solution (20). The presence of aromatic residues that are generally hydrophobic decreases the solubility of the peptides/proteins. Furthermore, as they can interact through Δstacking, these residues can orient the peptide-peptide interactions. From our data, we propose that aromatic-aromatic interactions drive such a self- assembly process. In the case of lanreotide, we propose that the DNal and the Tyr, due to their capacities through Δ interactions to orientate the self-assembly, represent the molecular ‘‘glue’’. Moreover, our experiments show that aromaticity is not enough. In particular, the behavior of the Tyr- Phe derivative compared with that of lanreotide raises the question of the specificity of this aromatic side chain. In recent preliminary Raman experiments we showed a very unusual modification of the Tyr environment in relation to its propensity to form H-bonds within the assemblies (57). Further experiments are required and have been undertaken to fully characterize the role of Tyr in the self-assembly process. However, this new molecular information supports the idea that at least part of the specificity of Tyr in lanreotide self- assembly is related to its capacity to act as an H-bound donor and/or acceptor. Of course, this conclusion does not exclude the involvement of the electron donor/acceptor properties of the aromatic cycle in the self-assembly process of lanreotide. Altogether, these results demonstrate that lanreotide self- assembly is driven by specific aromatic-aromatic interactions' [13].
Therefore it would seem of great scientific significance to create an equilibrium of positively and negatively charged amino acids together and observe how they interact under different conditions within living multicellular organisms. Considering the above in association with metal groups scientifically understood to be widely distributed in plants and soil across the globe, their photosynthetic qualities, reactions in splitting water [14, 15], synergy between Pt and Y, stereochemistry, the fact that platinum also appears to include classes of "species' [16, 17] and the order of command of amino acids and their influence on just about everything [13] modern technology offers exciting opportunities to understand their complex coevolutionary interrelationships.
Nevertheless, according to Li L, Yang XJ. (2015) "Microtubules are polar cylindrical polymers of α- and β-tubulin heterodimers (Fig. 1), and play important roles in maintaining cell morphology and regulating intracellular transport, mitosis, and cell migration [1–3]. Microtubules are an integral part of subcellular structures such as the cytoskeleton, mitotic spindles, centrioles, cilia and flagella [1–3]. To fulfill such diverse functions, at least three mechanisms are involved in the regulation: incorporation of different isoforms of α- and β-tubulin into microtubules, interaction with microtubule-associated proteins and plus-end-tracking proteins, and post-translational modifications of α- and β-tubulin [4–9]. (Under the secondary configuration this could also include γ, and δ, and platinum and Yttrium et al based nanotubes capable of more hardcore than usual bio-physiological and purging processes) The post-translational modifications include detyrosination (elimination of the C-terminal tyrosine residue from α-tubulin) [10], (Potentially relevant plausible explanation to the mysterious PAT1 protein, and Sm Domain and Sml Complex - C and N terminals) Δ2 modification (removal of the penultimate glutamate of α-tubulin after detyrosination) [11], acetylation [12, 13], phosphorylation [14], glutamylation [15] and glycylation [16]." [69] consistent with the biophysical facility of DMT and health issues associated with nutritional deprivation of it and similar substances. Based on the descriptions of [68, 69] Micro(nano and/or pico)tubules of appropriate forms subject to conscious covalent modifications of the secondary amino configuration also offers a plausible explanation for dynamic coordination of dendritic interfaces such as the NAc.
Since stumbling upon the influence of tryptamines upon Platinum nanotubes inspiring great interest in their bio-physiological roles particularly once consumed and learning of the essential role of microtubules I've now learned of carbon nanotubes.
Nano technology is, and arguably should be, a contentious subject. particularly regarding capitalistically inspired medical applications. However, understanding biophysics on a nanoscale is essential in understanding human health and dietary requirements. As the above is know and we have the technology to do so we should be investing in the health of our population to reduce the need for medical treatments and in the interests of ecological health too. According to Murjani, B.O., Kadu, P.S., Bansod, M. et al. (2022) Carbon Nanotubes (CNT) were discovered by Iijima in 1991 which, captivated the interest of many people due to their outstanding electrical, optical, thermal, and mechanical properties. Considered as most distinctive invention [5, 6] in nanotechnology CNTs include Five major biomedical applications being drug delivery, biosensors, bioimaging, tissue engineering, and cancer treatment which provides some incite into their qualities.
CNTs are formed by layers of carbon atoms mounted on each other in a rolled tube, in a hexagonal shape. These can usually be divided into two types. Single walled (SW)CNT range from 0.4 to 2 nm and typically occur as hexagonal packed bundles with distinct chemical properties of the side walls and end caps. Multi walled (MW)CNT consist of two or more walls and can serve as arterial structures to SWCNTs [71]. The CNTs have been synthesised up to 550mm long [72] and easily cross biological barriers and penetrate individual cells [71] which supports the hypothesis of natural functions such as purging via the appendix and other secretive channels. Interestingly, the most common sites of exposure to CNTs arethe pulmonary tract, skin, and gastrointestinal tract and animal studies found that CNTs entering the body via oral exposure reach the gastrointestinal tract in groups and are mostly excreted in feces without behavioral abnormality, change in body weight, or pathological abnormality of animals. [75] Amino-functionalized MWCNTs that bind to toxic siRNA sequences inhibiting tumor growth demonstrates that chemically functionalized CNTs can be used as transporters of siRNA in cancer therapy. With other studies showing that exposure to MWCNTs can up-regulate some miRNAs and down-regulating others [75] which could have pro’s and cons depending on the substrate and other variables.
MWCNT's are physically superior in terms of elasticity (≥ 1 TPA), tensile strength 65-93 GPa, double the thermal conductivity of a diamond and high electro-conductivity. and can withstand extreme temperatures increasing with atmospheric vacuum yet subjects to internal and external temp (presumably potentially regulated by layers). CNTs are hydrophobic (insoluble in water) [71] which in addition to DMT biochemical facility, is advantageous in facilitating stereo-inversion as a component of amino acids construction, coordination and disposal of natural Pt/Y CNTs as required.
Human consumption of platinum group nanoparticles is inevitable considering the uptake of Pt in plants like Lepidium Sativum which happens to be great for absorbing such particles from natural sources or contaminated soil off the side of the road [76, 79]. Therefore, in addition to the above and supporting Pt nanotubes due to it’s therapeutic value in imunomodulation regulation of inflammation and healing bones [77, 78] – I’ll certainly be using it as an admixture in my Ayahausca!
Pt, Ag, Au, or Hg and C have been used in the development of CNTs modified biosensors with a broad range of applications sue to their promotion of electron transfer reactions including glucose biosensors, protein sensors, nucleic acid sensors, immune-sensors, and infection sensors [71]. Whilst these have various por's and con's for man made applications they are also all of interest to natural bio-physiological intermolecular relationships.
As platinum and many, perhaps even all of the other substances can be consumed organically we'll disregard disputing such fact in relation to the following diagrams relating to six membered rings and "graphene" nanotube. Gang Liu, Xing-Qiu Chen, Bilu Liu, Wencai Ren, Hui-Ming Cheng (2021) do a great job of speaking to the complexity whilst breaking down the structure of nanotubes of countless molecular combinations in the following [73] with (n) Co2Sn2S2 depicted in "figure 1" (which is of a Pt transitional metal base).

"Figure 1. (a) Scheme of materials science and engineering research framework linked to different features. (b–p) The SMR structural unit from a bee’s honeycomb (b) in nature to 0D organic molecules (d and e), 2D monolayer or layered materials (f–i) to 3D crystalline materials (j–p) with the SMR structural layers bonded by vdW force, ionic, metallic or covalent bonds along the c-axis. (f–i) Four typical 2D monolayer SMR materials of graphene, h-BN, the 2H phase of MoS2 and Cu2 Si. (j–m) Four 3D SMR materials of Be/Mg, MgB2 , Ca3 P2 and Na3 Bi. The C6-fold SMR structural layers are composed of pure metallic Be, Mg and pure boron atoms for MgB2, respectively. In both Ca3P2 and Na3Bi, their individual SMR layers comprise Ca/P and Na/Bi atoms, respectively, under the C3-fold rotation symmetry. (n–p) The 3D SMR materials of WC, Co3Sn2S2 and Bi2Se3 in which their C3-fold rotational SMR structural layers comprise W/C, Co/Sn and Bi/Se atoms, respectively. Their specified symmetry, space group and point group are summarized in Table 1." Image and discription from [73].

Image and discription from [73].
The two figures above imediately have similarities with the motifs in the SM domains. Particularly in relation to (n) Co2Sn2S2 depicted in "figure 1" (which is of a Pt transitional metal base). Regardless of material for differenct substrates, cells and nanotubes there are strong arguments such as stability and borader psyiological facility supporting the notion "too much is better than not enough" [70, 73]. Obviously this depends upon substances and routs of administrations etc. Whilst DMT is particularly forgiving "too much" Pt and Y consumed orrally likely has considerable leway potentially relient upon enough DMT and supporting substrate to get in and out and the job done right without leaving a half finished harmful mess behind. However, whilst adequate quantities will be required for certain tasks. consumed orally is likely that the system prioritises how much "nutrition" it has towards required maintenance. Once again regulation of pH has proven influential with a targeted drug delivery system developed by Zhang et al. using SWCNTs to deliver an anticancer drug at, you guessed it, a physiological pH of 7.4. Studies with gold nanoparticles found N-methyl 4-vinyl pyridine iodide and methacrylic acid which significantly enhanced water compatibility [73].
This additional conclusion quote from the above study leads into this reasonable well...
"4, as only pp stacking is involved in this interaction.
The extreme sensitivity of the self-assembly processes of lanreotide to the molecular sequence of the peptide provides information that can be related to the molecular parameters driving amyloid-related self-assemblies. Finally, this work also describes some of the parameters necessary for the bottom-up design of new peptide families that self-assemble into tunable nanotubule or fibril architectures."
As previously speculated could it in-fact be a case of turning these little fuckers inside out? Ha. I've just noticed they don't include the other 4 possible "dynamic" ranges in each "configuration"!?! Although it is said only left handed aminos are used on earth the countless possible combinations above offer a temporarily "racemic" [59] configuration only possible with conscious coordination of coevolution of all aspects of life throughout space and time. Having said this I am unsure I am unsure if aromatic rings is 100% universal in any direction or whether it has limitations in particular directions!?!

Interestingly the phosphate ion looks to match with these suckers perfectly and I can envisage ways in which it fits within nanotubes. Whilst I’m not entirely sure on how it’s amorousness works the R / S configuration could possibly be applied to the L / D amino configurations, reversal of mRNA protein actions and other processes associated with phosphorylation.
So where does that leave the original hypothesis? "N,N-Dimethyltryptamine (DMT) & Other "Forbidden Fruit" - Central Nervous System Specific Nutrients." That is complicated. Regardless of what you call them and their temporary nature the available evidence srongly affirms that DMT "and other forbidden fruit" are essential componants of a healthy diet. The lack of half-life is more like food. However, there is something very intimate and almost sexual in what would presumably require at least two sets (or subdivisions) of conciousness to harmonously coordinate such complex combinations of interrelationships. The crystal structures are evidence of seperation between distinctly strict and divergent configurations. Assuming it is in fact correct that "both" are conscious, and perhaps Triptophan acts as the doorway acompanying DMT through space and time to a collective conciousness then back again elsewhere contributing to global evolution. I'm not entirely sure what this how this fits with the definitions of indogenous exdoginous as it almost seems they are both exdoginous running through us rather than being produced by us. Finally, in addition to evidence of countless pathways supporting the secondary amino configuration hypothesis - it has been possible to identify some testing considerations for the considerations for Blood/Urine Analysis - Metabolic Implications of Psychedelics / Forbidden Fruit upon DRUG Saturation of Universal Asylum's Metabolic Implications of "Psychedelics / Forbidden Fruit" upon DRUG Saturation - Planning Document. However, the exact nature of available scientific analysis options is well beyond my professional scope as a "Social Worker".
Continued in
References
PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 4525689, N,N-dimethyltryptamine; [cited 2024 July 16]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/6089
Achsel T, Stark H, Lührmann R. The Sm domain is an ancient RNA-binding motif with oligo(U) specificity. Proc Natl Acad Sci U S A. 2001 Mar 27;98(7)/3685-9. doi/ 10.1073/pnas.071033998. Epub 2001 Mar 20. PMID/ 11259661; PMCID/ PMC31112.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC31112/
Chowdhury A, Kalurupalle S, Tharun S. Pat1 contributes to the RNA binding activity of the Lsm1-7-Pat1 complex. RNA. 2014 Sep;20(9):1465-75. doi: 10.1261/rna.045252.114. Epub 2014 Jul 17. PMID: 25035297; PMCID: PMC4138329. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4138329/
Metzner L, Kottra G, Neubert K, Daniel H, Brandsch M. Serotonin, L-tryptophan, and tryptamine are effective inhibitors of the amino acid transport system PAT1. FASEB J. 2005 Sep;19(11):1468-73. doi: 10.1096/fj.05-3683com. PMID: 16126914. https://pubmed.ncbi.nlm.nih.gov/16126914/
Aline Marnef, Nancy Standart; Pat1 proteins: a life in translation, translation repression and mRNA decay. Biochem Soc Trans 1 December 2010; 38 (6): 1602–1607. doi: https://doi.org/10.1042/BST0381602
Marija Liutkute, Manisankar Maiti, Ekaterina, Samatova, Jörg Enderlein, Marina V Rodnina (2020) Gradual compaction of the nascent peptide during cotranslational folding on the ribosome eLife 9:e60895 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7593090/#:~:text=The%20rate%20constants%20of%20ATTO655,D%2C%20which%20we%20call%20dynamic.
Maguire BA, Beniaminov AD, Ramu H, Mankin AS, Zimmermann RA. A protein component at the heart of an RNA machine: the importance of protein l27 for the function of the bacterial ribosome. Mol Cell. 2005 Nov 11;20(3):427-35. doi: 10.1016/j.molcel.2005.09.009. PMID: 16285924. https://pubmed.ncbi.nlm.nih.gov/16285924/
Xu, D., Yang, L., Wang, Y. et al. Proteins enriched in charged amino acids control the formation and stabilization of selenium nanoparticles in Comamonas testosteroni S44.Sci Rep 8, 4766 (2018). https://doi.org/10.1038/s41598-018-23295-5 https://www.nature.com/articles/s41598-018-23295-5#citeas
Sun Y, Shin H, Wang F, Tian B, Chiang CW, Liu S, Li X, Wang Y, Tang L, Goddard WA 3rd, Ding M. Highly Selective Electrocatalytic Oxidation of Amines to Nitriles Assisted by Water Oxidation on Metal-Doped α-Ni(OH)2. J Am Chem Soc. 2022 Aug 24;144(33):15185-15192. doi: 10.1021/jacs.2c05403. Epub 2022 Aug 10. PMID: 35948416. https://pubmed.ncbi.nlm.nih.gov/35948416/
PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 4525689, N,N-dimethyltryptaminium; [cited 2024 July 16]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/N_N-dimethyltryptaminium
Cucchiaro A, Scherfler A, Corinti D, Berden G, Oomens J, Wurst K, Gust R, Crestoni ME, Kircher B, Cziferszky M. Amino Acids as Chelating Ligands for Platinum: Enhanced Stability in an Aqueous Environment Promoted by Biocompatible Molecules. J Med Chem. 2023 Nov 23;66(22):15256-15268. doi: 10.1021/acs.jmedchem.3c01340. Epub 2023 Nov 8. PMID: 37937969; PMCID: PMC10683014. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10683014/
ARUNAVA MISRA, SK RAJIBUL HAQUE and MOHABUL A MONDAL* Department of Chemistry, Jadavpur University, Kolkata 700032, Yttrium nitrate promoted selective cyanoethylation of amines. J. Chem. Sci. (2023) 135:57 .https://doi.org/10.1007/s12039-023-02173-2Sadhana(0123456789().,-volV)FT3](0123456 789().,-volV)
https://www.ias.ac.in/article/fulltext/jcsc/135/0057
Valéry C, Pouget E, Pandit A, Verbavatz JM, Bordes L, Boisdé I, Cherif-Cheikh R, Artzner F, Paternostre M. Molecular origin of the self-assembly of lanreotide into nanotubes: a mutational approach. Biophys J. 2008 Mar 1;94(5):1782-95. doi: 10.1529/biophysj.107.108175. Epub 2007 Nov 9. PMID: 17993497; PMCID: PMC2242760. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2242760/
Ivana Maksimovic, Rudolf Kastori, Marina Putnik-Delic, Milan Borišev,
Effect of yttrium on photosynthesis and water relations in young maize plants,
Journal of Rare Earths, Volume 32, Issue 4, 2014, Pages 372-378, ISSN 1002-0721,
https://doi.org/10.1016/S1002-0721(14)60080-6.
(https://www.sciencedirect.com/science/article/pii/S1002072114600806)
Ruoqing Yao, Yanxi Li, Yang Chen, Boran Xu, Changhui Chen*, Chunxi Zhang* Rare-Earth Elements Can Structurally and Energetically Replace the Calcium in a Synthetic Mn4CaO4-Cluster Mimicking the Oxygen-Evolving Center in Photosynthesis, Journal of the American Chemical Society, Vol 143/Issue 42 Article, https://pubs.acs.org/doi/10.1021/jacs.1c09085
Yohana Martínez Galeano, Martín Mizrahi, José M. Ramallo-López, Sergio Moreno, Laura Cornaglia, Ana M. Tarditi, Identification and localization of Pt species in Pt-NaA zeolite catalysts prepared by direct synthesis, Catalysis Communications, Volume 172, 2022, 106558, ISSN 1566-7367, (https://www.sciencedirect.com/science/article/pii/S1566736722001637)
Jiang L, Tian C, Li Y, Si R, Du M, Li X, Guo L, Li L. NaCl-Templated Ultrathin 2D-Yttria Nanosheets Supported Pt Nanoparticles for Enhancing CO Oxidation Reaction. Nanomaterials (Basel). 2022 Jul 5;12(13):2306. doi: 10.3390/nano12132306. PMID: 35808141; PMCID: PMC9268161. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9268161/
Kaur, Ramanpreet & Vikas,. (2017). From nitrogen inversion in amines to stereoinversion in aminium salts: role of a single water molecule. Theoretical Chemistry Accounts. 136. 10.1007/s00214-017-2090-2. https://www.researchgate.net/publication/316265846_From_nitrogen_inversion_in_amines_to_stereoinversion_in_aminium_salts_role_of_a_single_water_molecule Khan
Claire M. Smathers, Aaron R. Robart, The mechanism of splicing as told by group II introns: Ancestors of the spliceosome, Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms, Volume 1862, Issues 11–12, 2019, 194390, ISSN 1874-9399, https://doi.org/10.1016/j.bbagrm.2019.06.001.
Lai, Yuekun & Jiaojiao, Goog & Lin, Changjian. (2012). Self-organized TiO2 nanotube arrays with uniform platinum nanoparticles for highly efficient water splitting. International Journal of Hydrogen Energy. 37. 6438-6446.. 10.1016/j.ijhydene.2012.01.078. https://www.sciencedirect.com/science/article/abs/pii/S0360319912001280
Messerschmidt J, Alt F, Tölg G. Detection of platinum species in plant material. Electrophoresis. 1995 May;16(5):800-3. doi: 10.1002/elps.11501601131. PMID: 7588565. https://pubmed.ncbi.nlm.nih.gov/7588565/
Collins BM, Cubeddu L, Naidoo N, Harrop SJ, Kornfeld GD, Dawes IW, Curmi PM, Mabbutt BC. Homomeric ring assemblies of eukaryotic Sm proteins have affinity for both RNA and DNA. Crystal structure of an oligomeric complex of yeast SmF. J Biol Chem. 2003 May 9;278(19):17291-8. doi: 10.1074/jbc.M211826200. Epub 2003 Mar 4. PMID: 12618433. https://www.jbc.org/article/S0021-9258(19)58462-9/fulltext
PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 9060, D-tryptophan [Cited 01st Sept 2024] https://pubchem.ncbi.nlm.nih.gov/compound/D-tryptophan
Nogueira, Margareth & Golbert, Daiane & Menezes, Richardson & Almeida, Raíssa & Galvão-Coelho, Nicole & Siroky, Andressa & Lima, Thiago & Peixoto, Helton & Leao, Katarina & Leao, Richardson. (2024). Serotonergic psychedelic 5-MeO-DMT alters plasticity-related gene expression and generates anxiolytic effects in stressed mice. Molecular Psychiatry. 1-11. 10.1038/s41380-024-02655-w. https://www.researchgate.net/publication/382025962_Serotonergic_psychedelic_5-MeO-DMT_alters_plasticity-related_gene_expression_and_generates_anxiolytic_effects_in_stressed_mice
Farías-Rico JA, Mourra-Díaz CM. A Short Tale of the Origin of Proteins and Ribosome Evolution. Microorganisms. 2022 Oct 26;10(11)-2115. doi- 10.3390/microorganisms10112115. PMID- 36363706; PMCID- PMC9694802..pdf https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9694802/
Kumar D, Steele EJ, Wickramasinghe NC. Preface: The origin of life and astrobiology. Adv Genet. 2020;106:xv-xviii. doi: 10.1016/S0065-2660(20)30037-7. PMID: 33081930; PMCID: PMC7568464. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7568464/
Makarov, Mikhail & Sanchez Rocha, Alma & Kryštůfek, Robin & Cherepashuk, Ivan & Dzmitruk, Volha & Chernovets, Tatsiana & Faustino, Anneliese & Lebl, Michal & Fujishima, Kosuke & Fried, Stephen & Hlouchova, Klara. (2023). Early Selection of the Amino Acid Alphabet Was Adaptively Shaped by Biophysical Constraints of Foldability. Journal of the American Chemical Society. 145. 10.1021/jacs.2c12987. https://pubs.acs.org/doi/full/10.1021/jacs.2c12987
Bywater RP. Why twenty amino acid residue types suffice(d) to support all living systems. PLoS One. 2018 Oct 15;13(10):e0204883. doi: 10.1371/journal.pone.0204883. PMID: 30321190; PMCID: PMC6188899. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6188899/
Wang C, Vidal B, Sural S, Loer C, Aguilar GR, Merritt DM, Toker IA, Vogt MC, Cros C, Hobert O. A neurotransmitter atlas of C. elegans males and hermaphrodites. bioRxiv [Preprint]. 2024 Jun 7:2023.12.24.573258. doi: 10.1101/2023.12.24.573258. PMID: 38895397; PMCID: PMC11185579. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11185579/
Horie A, Tomita T, Saiki A, Kono H, Taka H, Mineki R, Fujimura T, Nishiyama C, Kuzuyama T, Nishiyama M. Discovery of proteinaceous N-modification in lysine biosynthesis of Thermus thermophilus. Nat Chem Biol. 2009 Sep;5(9):673-9. doi: 10.1038/nchembio.198. Epub 2009 Jul 20. PMID: 19620981. https://pubmed.ncbi.nlm.nih.gov/19620981/
EcoCyc Website, 2024 compound class : an aminated amino group donor, Accessed 3rd Sep 2024. https://biocyc.org/compound?orgid=ECOLI&id=Amine-Donors
PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary: Lysine: https://pubchem.ncbi.nlm.nih.gov/compound/5962
Al Temimi, A.H.K., Amatdjais-Groenen, H.I.V., Reddy, Y.V. et al. The nucleophilic amino group of lysine is central for histone lysine methyltransferase catalysis. Commun Chem 2, 112 (2019). https///doi.org/10.1038/s42004-019-0210-8
Haruna Sugahara, Cornelia Meinert, Laurent Nahon, Nykola C. Jones, Søren V. Hoffmann, Kenji Hamase, Yoshinori Takano, Uwe J. Meierhenrich, d-Amino acids in molecular evolution in space – Absolute asymmetric photolysis and synthesis of amino acids by circularly polarized light, Biochimica et Biophysica Acta (BBA) - Proteins and Proteomics, Volume 1866, Issue 7, 2018, Pages 743-758, ISSN 1570-9639, (https://www.sciencedirect.com/science/article/pii/S1570963918300050)
León-Ponte M, Ahern GP, O'Connell PJ. Serotonin provides an accessory signal to enhance T-cell activation by signaling through the 5-HT7 receptor. Blood. 2007 Apr 15;109(8):3139-46. doi: 10.1182/blood-2006-10-052787. Epub 2006 Dec 7. PMID: 17158224; PMCID: PMC1852236. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1852236/
Zhang, D., Lape, R., Shaikh, S.A. et al. Modulatory mechanisms of TARP γ8-selective AMPA receptor therapeutics. Nat Commun 14, 1659 (2023). https://doi.org/10.1038/s41467-023-37259-5 https://www.nature.com/articles/s41467-023-37259-5
Federico Miguez-Cabello, Nuria Sánchez-Fernández, Natalia Yefimenko, Xavier Gasull, Esther Gratacòs-Batlle, David Soto (2020) AMPAR/TARP stoichiometry differentially modulates channel properties eLife 9:e53946 https://doi.org/
Manfredi et al. Inventors: Paolo L. Manfredi, Miami Beach ,FL (US); Charles E. Inturisi, NewYork, NY(US); Sara De Martin,Vigonza (IT); Andrea Mattarei, Perarolodi Vigonza (IT); Maurizio Rolando, Genova (IT); Giovanni Giordano, Genova (IT); Claudia Lodovichi, Padova(IT); Paola Brun, Padova (IT); Marco Papagalo, NewYork, NY (US); Franco Foli, Milane (IT); Andrea Alimonti, Belinzona (CH); Jacopo Sgrignani, Almenno San Salvatore (IT); Andrea Cavali, Zurich (CH) Aplicant: Arbormentis LLC, Miami Beach, FL (US) COMPOSITIONSANDMETHODSOFUSE COMPRISING SUBSTANCES WITH NEURAL PLASTICITY ACTIONS ADMINISTERED AT NON -PSYCHEDELIC /PSYCHOTOMIMETIC DOSAGES AND FORMULATIONS, United States Pub.No.: US202/0143051A1; May 12,2022
Flores-Soto ME, Chaparro-Huerta V, Escoto-Delgadillo M, Vazquez-Valls E, González-Castañeda RE, Beas-Zarate C. Estructura y función de las subunidades del receptor a glutamato tipo NMDA [Structure and function of NMDA-type glutamate receptor subunits]. Neurologia. 2012 Jun;27(5):301-10. Spanish. doi: 10.1016/j.nrl.2011.10.014. Epub 2012 Jan 2. PMID: 22217527. https://www.elsevier.es/pt-revista-neurologia-english-edition--495-articulo-structure-function-nmda-type-glutamate-receptor-S2173580812000739#bib0090
Autry AE, Monteggia LM. Brain-derived neurotrophic factor and neuropsychiatric disorders. Pharmacol Rev. 2012 Apr;64(2):238-58. doi: 10.1124/pr.111.005108. Epub 2012 Mar 8. PMID: 22407616; PMCID: PMC3310485. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3310485/#:~:text=Brain%20derived%20neurotrophic%20factor%20(BDNF,widely%20implicated%20in%20psychiatric%20diseases.
Panwar, Vivek & Singh, Aishwarya & Bhatt, Manini & Tonk, Rajiv & Azizov, Shavkatjon & Raza, Agha & Sengupta, Shinjinee & Kumar, Deepak & Garg, Manoj. (2023). Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduction and Targeted Therapy. 8. 375. 10.1038/s41392-023-01608-z. https://www.researchgate.net/publication/374371662_Multifaceted_role_of_mTOR_mammalian_target_of_rapamycin_signaling_pathway_in_human_health_and_disease
Jednačak, Tomislav, Ivana Mikulandra, and Predrag Novak. 2020. "Advanced Methods for Studying Structure and Interactions of Macrolide Antibiotics" International Journal of Molecular Sciences 21, no. 20: 7799. https://doi.org/10.3390/ijms21207799 https://www.mdpi.com/1422-0067/21/20/7799
Litman, Y., Chiang, KY., Seki, T. et al. Surface stratification determines the interfacial water structure of simple electrolyte solutions. Nat. Chem. 16, 644–650 (2024). https://doi.org/10.1038/s41557-023-01416-6 https://www.nature.com/articles/s41557-023-01416-6#citeas
Ralls, E 2024 ,Water molecule discovery will force textbooks to be rewritten, Earth.com. [viewed, 21 Sep 2024]. https://www.earth.com/news/new-discovery-water-molecules-will-force-chemistry-textbooks-to-be-rewritten/?fbclid=IwY2xjawFaYtlleHRuA2FlbQIxMQABHcisfcJV_8h733HLkCwrZg4YgZ1L-eEmffH4AvrFFSalBYgcLZ2OJYV8Ww_aem_746ssPVguvae2RojgbTIkQ
Egger, K., Aicher, H.D., Cumming, P. et al. Neurobiological research on N,N-dimethyltryptamine (DMT) and its potentiation by monoamine oxidase (MAO) inhibition: from ayahuasca to synthetic combinations of DMT and MAO inhibitors. Cell. Mol. Life Sci.81, 395 (2024). https://doi.org/10.1007/s00018-024-05353-6 https://link.springer.com/article/10.1007/s00018-024-05353-6?fbclid=IwY2xjawFciWtleHRuA2FlbQIxMAABHdtxaOC6uSgZLmxoiVQhk39phRN11GmcG3hQn8fajs2XRtwqq7vQTtnzkQ_aem_aBgBntMXjlL61Qsfp0Fn8w
Wang C, Vidal B, Sural S, Loer C, Aguilar GR, Merritt DM, Toker IA, Vogt MC, Cros C, Hobert O. A neurotransmitter atlas of C. elegans males and hermaphrodites. bioRxiv [Preprint]. 2024 Jun 7:2023.12.24.573258. doi: 10.1101/2023.12.24.573258. PMID: 38895397; PMCID: PMC11185579. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC11185579/
Yusupova, Gulnara & Yusupov, Marat. (2017). Crystal structure of eukaryotic ribosome and its complexes with inhibitors. Philosophical Transactions of the Royal Society B: Biological Sciences. 372. 20160184. 10.1098/rstb.2016.0184. https://www.researchgate.net/publication/313122996_Crystal_structure_of_eukaryotic_ribosome_and_its_complexes_with_inhibitors/download
Panwar V, Singh A, Bhatt M, Tonk RK, Azizov S, Raza AS, Sengupta S, Kumar D, Garg M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct Target Ther. 2023 Oct 2;8(1):375. doi: 10.1038/s41392-023-01608-z. PMID: 37779156; PMCID: PMC10543444. https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/37779156/
Plein LM, Rittner HL. Opioids and the immune system - friend or foe. Br J Pharmacol. 2018 Jul;175(14):2717-2725. doi: 10.1111/bph.13750. Epub 2017 Mar 23. PMID: 28213891; PMCID: PMC6016673. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6016673/
Mikati MO, Klein RS. Fragile-like regulatory T cells modulate opioid withdrawal in humans and mice. Immunity. 2023 Feb 14;56(2):237-239. doi: 10.1016/j.immuni.2023.01.021. PMID: 36792570; PMCID: PMC10867865. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC10867865/
Pahwa R, Goyal A, Jialal I. Chronic Inflammation. [Updated 2023 Aug 7]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK493173/
Rouse BT, Sehrawat S. Immunity and immunopathology to viruses: what decides the outcome? Nat Rev Immunol. 2010 Jul;10(7):514-26. doi: 10.1038/nri2802. PMID: 20577268; PMCID: PMC3899649. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3899649/
Szabo A, Kovacs A, Frecska E, Rajnavolgyi E. Psychedelic N,N-dimethyltryptamine and 5-methoxy-N,N-dimethyltryptamine modulate innate and adaptive inflammatory responses through the sigma-1 receptor of human monocyte-derived dendritic cells. PLoS One. 2014 Aug 29;9(8):e106533. doi: 10.1371/journal.pone.0106533. PMID: 25171370; PMCID: PMC4149582. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4149582/
Szabo A, Frecska E. Dimethyltryptamine (DMT): a biochemical Swiss Army knife in neuroinflammation and neuroprotection? Neural Regen Res. 2016 Mar;11(3):396-7. doi: 10.4103/1673-5374.179041. PMID: 27127466; PMCID: PMC4828992. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4828992/
Rudin D, Areesanan A, Liechti ME, Gründemann C. Classic psychedelics do not affect T cell and monocyte immune responses. Front Psychiatry. 2023 Jan 20;14:1042440. doi: 10.3389/fpsyt.2023.1042440. PMID: 36741125; PMCID: PMC9895091. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9895091/
Castro, DC & Bruchas, MR. 2019. A Motivational and Neuropeptidergic Hub: Anatomical and Functional Diversity within the Nucleus Accumbens Shell, Neuron, Volume 102, Issue 3, 2019, Pages 529-552, ISSN 0896-6273, https://www.sciencedirect.com/science/article/pii/S0896627319302107
Diering GH, Mills F, Bamji SX, Numata M. Regulation of dendritic spine growth through activity-dependent recruitment of the brain-enriched Na⁺/H⁺ exchanger NHE5. Mol Biol Cell. 2011 Jul 1;22(13):2246-57. doi: 10.1091/mbc.E11-01-0066. Epub 2011 May 5. PMID: 21551074; PMCID: PMC3128527. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3128527/
Helen M. Gooch, Tobias Bluett, Madhusoothanan B. Perumal, Hong D. Vo, Lee N. Fletcher, Jason Papacostas, Rosalind L. Jeffree, Martin Wood, Michael J. Colditz, Jason McMillen, Tony Tsahtsarlis, Damian Amato, Robert Campbell, Lisa Gillinder, Stephen R. Williams, High-fidelity dendritic sodium spike generation in human layer 2/3 neocortical pyramidal neurons, Cell Reports, Volume 41, Issue 3, 2022, 111500, ISSN 2211-1247, (https://www.sciencedirect.com/science/article/pii/S221112472201350X)
The Astrophysics & Astrochemistry Lab at Nasa Ames Research Centre Website . Amoino Acides and Their Production during the Photolysis of Astrophysically Relevant Ices. Viewed 28th September 2024. https://www.astrochem.org/sci/Amino_Acids.php
Parker, M. 2024, DMT(+), Serotonin & Tryptophan(s) Brønsted Bondage, π-Systems, Quantum-Tunneling & Complete Reversible Hydrolytic Splicing: Coevolution of Group II Introns & RNA Viruses... OMG Filthy Little Whores!!!, Universal Asylum https://www.universalasylum.com.au/post/quantum-biophysics-for-fucken-retards-n-n-dimethyltriptamine-the-key-component-of-regular-a-health
Elevated Tryptase: Conditions and Pitfalls, Pongdee, Thanai et al. The Journal of Allergy and Clinical Immunology: In Practice, Volume 10, Issue 9, 2436 - 2437 https://www.jaci-inpractice.org/article/S2213-2198(22)00650-X/fulltext
Fong M, Crane JS. Histology, Mast Cells. [Updated 2023 May 1]. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2024 Jan-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK499904/
Robinson, C. Mast Cells: Mast Cell Function, Origin and Related Conditions, Discover the secrets of mast cells and their role in the immune system, Article , Published: February 15, 2024, Last Updated: March 19, 2024, https://www.technologynetworks.com/immunology/articles/mast-cells-mast-cell-function-origin-and-related-conditions-382775
Krystel-Whittemore Melissa , Dileepan Kottarappat N. , Wood John G. Mast Cell: A Multi-Functional Master Cell, Frontiers in Immunology, VOL 6, 2016 DOI: 10.3389/fimmu.2015.00620. ISSN:L 1664-3224
https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2015.00620
Kamide, Yosuke et al. 2016. Acidic Conditions Regulate Mast Cell Migration and Fc Epsilon RI–Mediated Cytokine Production. Journal of Allergy and Clinical Immunology, Volume 137, Issue 2, AB75a. https://www.jacionline.org/article/S0091-6749(15)01995-8/fulltext
Breitling S, Hui Z, Zabini D, Hu Y, Hoffmann J, Goldenberg NM, Tabuchi A, Buelow R, Dos Santos C, Kuebler WM. The mast cell-B cell axis in lung vascular remodeling and pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2017 May 1;312(5):L710-L721. doi: 10.1152/ajplung.00311.2016. Epub 2017 Feb 24. PMID: 28235950. https://pubmed.ncbi.nlm.nih.gov/28235950/
Harvima IT, Harvima RJ, Eloranta TO, Fräki JE. The allosteric effect of salt on human mast cell tryptase. Biochim Biophys Acta. 1988 Sep 21;956(2):133-9. doi: 10.1016/0167-4838(88)90259-2. PMID: 3048411. https://pubmed.ncbi.nlm.nih.gov/3048411/
Li L, Yang XJ. Tubulin acetylation: responsible enzymes, biological functions and human diseases. Cell Mol Life Sci. 2015 Nov;72(22):4237-55. doi: 10.1007/s00018-015-2000-5. Epub 2015 Jul 31. PMID: 26227334; PMCID: PMC11113413. https://www.ncbi.nlm.nih.gov/pmc/articles/pmid/26227334/
Sanyal C, Pietsch N, Ramirez Rios S, Peris L, Carrier L, Moutin MJ. The detyrosination/re-tyrosination cycle of tubulin and its role and dysfunction in neurons and cardiomyocytes. Semin Cell Dev Biol. 2023 Mar 15;137:46-62. doi: 10.1016/j.semcdb.2021.12.006. Epub 2021 Dec 17. PMID: 34924330. https://www.sciencedirect.com/science/article/pii/S1084952121003141?via%3Dihub
Carsten Janke, Guillaume Montagnac, Causes and Consequences of Microtubule Acetylation, Current Biology, Volume 27, Issue 23, 2017, Pages R1287-R1292, ISSN 0960-9822, (https://www.sciencedirect.com/science/article/pii/S0960982217313817)
Murjani, B.O., Kadu, P.S., Bansod, M. et al. Carbon nanotubes in biomedical applications: current status, promises, and challenges. Carbon Lett. 32, 1207–1226 (2022). https://doi.org/10.1007/s42823-022-00364-4 https://link.springer.com/article/10.1007/s42823-022-00364-4#citeas
Zhang R, Zhang Y, Zhang Q, Xie H, Qian W, Wei F. Growth of half-meter long carbon nanotubes based on Schulz-Flory distribution. ACS Nano. 2013 Jul 23;7(7):6156-61. doi: 10.1021/nn401995z. Epub 2013 Jul 8. Erratum in: ACS Nano. 2014 Mar 25;8(3):3097. PMID: 23806050. https://pubmed.ncbi.nlm.nih.gov/23806050/
Gang Liu, Xing-Qiu Chen, Bilu Liu, Wencai Ren, Hui-Ming Cheng, Six-membered-ring inorganic materials: definition and prospects, National Science Review, Volume 8, Issue 1, January 2021, nwaa248, https://academic.oup.com/nsr/article/8/1/nwaa248/5912469
Claire M. Smathers, Aaron R. Robart, The mechanism of splicing as told by group II introns: Ancestors of the spliceosome, Biochimica et Biophysica Acta (BBA) - Gene Regulatory Mechanisms, Volume 1862, Issues 11–12, 2019, 194390, ISSN 1874-9399, https://doi.org/10.1016/j.bbagrm.2019.06.001.
Zhang C, Wu L, de Perrot M, Zhao X. Carbon Nanotubes: A Summary of Beneficial and Dangerous Aspects of an Increasingly Popular Group of Nanomaterials. Front Oncol. 2021 Jul 27;11:693814. doi: 10.3389/fonc.2021.693814. PMID: 34386422; PMCID: PMC8353320.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8353320/
Asztemborska M, Steborowski R, Kowalska J, Bystrzejewska-Piotrowska G. Accumulation of Platinum Nanoparticles by Sinapis alba and Lepidium sativum Plants. Water Air Soil Pollut. 2015;226(4):126. doi: 10.1007/s11270-015-2381-y. Epub 2015 Apr 1. PMID: 25859065; PMCID: PMC4381038. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4381038/
Dixit Jr Iii V, Kumar I, Palandurkar K, Giri R, Giri K. Lepidium sativum: Bone healer in traditional medicine, an experimental validation study in rats. J Family Med Prim Care. 2020 Feb 28;9(2):812-818. doi: 10.4103/jfmpc.jfmpc_761_19. PMID: 32318426; PMCID: PMC7113932. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7113932/
Vazifeh S, Kananpour P, Khalilpour M, Eisalou SV, Hamblin MR. Anti-inflammatory and Immunomodulatory Properties of Lepidium sativum. Biomed Res Int. 2022 Jul 23;2022:3645038. doi: 10.1155/2022/3645038. PMID: 35937400; PMCID: PMC9348929. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9348929/
Whiteley JD, Murray F. Anthropogenic platinum group element (Pt, Pd and Rh) concentrations in road dusts and roadside soils from Perth, Western Australia. Sci Total Environ. 2003 Dec 30;317(1-3):121-35. doi: 10.1016/S0048-9697(03)00359-0. PMID: 14630416. https://pubmed.ncbi.nlm.nih.gov/14630416/
Nian-Dong Mao, Yang Ye, Xiao-Tao Zhuo, Xiang-Yang Ye, Tian Xie, Recent advances of the site-specific direct methylation on aromatic rings, Tetrahedron, Volume 96, 2021, 132402, ISSN 0040-4020, (https://www.sciencedirect.com/science/article/pii/S004040202100661X)
Warren W. Tryon, Chapter 3 - Core Network Principles: The Explanatory Nucleus, Editor(s): Warren W. Tryon, Cognitive Neuroscience and Psychotherapy, Academic Press, 2014, Pages 125-222, ISBN 9780124200715, X(https://www.sciencedirect.com/science/article/pii/B978012420071500003X)
Fish, M.S.; Johnson, N.M.; Horning, E.C., 1956, t-Amine Oxide Rearrangements. N,N-Dimethyltryptamine Oxide. Journal of the American Chemical Society Aug 1956 78(5) 3668-3671 https://bibliography.maps.org/bibliography/default/resource/13234
National Center for Biotechnology Information (2024). PubChem Compound Summary for CID 29936, Ferric cation. Retrieved October 18, 2024 from https://pubchem.ncbi.nlm.nih.gov/compound/Ferric-cation.
PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-. PubChem Compound Summary for CID 5326976, 4,5,6,7-Tetrabromo-N,N-dimethyl-1H-benzimidazol-2-amine; [cited 2024 Oct. 18]. Available from: https://pubchem.ncbi.nlm.nih.gov/compound/dmat
Crysten E. Blaby-Haas, Sabeeha S. Merchant, The ins and outs of algal metal transport, Biochimica et Biophysica Acta (BBA) - Molecular Cell Research, Volume 1823, Issue 9, 2012, Pages 1531-1552, ISSN 0167-4889, (https://www.sciencedirect.com/science/article/pii/S0167488912001012)
Alan L. Balch, Krzysztof Winkler, Electrochemistry of fullerene/transition metal complexes: Three decades of progress, Coordination Chemistry Reviews, Volume 438, 2021, 213623, ISSN 0010-8545, https://www.sciencedirect.com/science/article/pii/S001085452030758X)
Wu X, Cañamares MV, Kakoulli I, Sanchez-Cortes S. Chemical Characterization and Molecular Dynamics Simulations of Bufotenine by Surface-Enhanced Raman Scattering (SERS) and Density Functional Theory (DFT). J Phys Chem Lett. 2022 Jun 30;13(25):5831-5837. doi: 10.1021/acs.jpclett.2c01300. Epub 2022 Jun 21. PMID: 35726975; PMCID: PMC9251765.https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9251765/
Alexandra Zakharova, Daria Tashyreva, Anzhelika Butenko, Jorge Morales, Andreu Saura, Michaela Svobodová, Gereon Poschmann, Satish Nandipati, Alena Zakharova, David Noyvert, Ondřej Gahura, Jiří Týč, Kai Stühler, Alexei Y. Kostygov, Eva C.M. Nowack, Julius Lukeš, Vyacheslav Yurchenko,
A neo-functionalized homolog of host transmembrane protein controls localization of bacterial endosymbionts in the trypanosomatid Novymonas esmeraldas, Current Biology, Volume 33, Issue 13, 2023, Pages 2690-2701.e5, ISSN 0960-9822, https://doi.org/10.1016/j.cub.2023.04.060.(https://www.sciencedirect.com/science/article/pii/S0960982223005420)
Ryan M.R. Gawryluk, Symbiosis: A duplicated host protein controlling a nascent mutualism,
Current Biology, Volume 33, Issue 13, 2023, Pages R712-R715, ISSN 0960-9822, https://doi.org/10.1016/j.cub.2023.05.052.
(https://www.sciencedirect.com/science/article/pii/S096098222300684X)
Zakharova A, Saura A, Butenko A, Podešvová L, Warmusová S, Kostygov AY, Nenarokova A, Lukeš J, Opperdoes FR, Yurchenko V. A New Model Trypanosomatid, Novymonas esmeraldas: Genomic Perception of Its "Candidatus Pandoraea novymonadis" Endosymbiont. mBio. 2021 Aug 31;12(4):e0160621. doi: 10.1128/mBio.01606-21. Epub 2021 Aug 17. PMID: 34399629; PMCID: PMC8406214. https://pubmed.ncbi.nlm.nih.gov/34399629/
Volkov OA, Brockway AJ, Wring SA, Peel M, Chen Z, Phillips MA, De Brabander JK. Species-Selective Pyrimidineamine Inhibitors of Trypanosoma brucei S-Adenosylmethionine Decarboxylase. J Med Chem. 2018 Feb 8;61(3):1182-1203. doi: 10.1021/acs.jmedchem.7b01654. Epub 2018 Jan 5. PMID: 29271204; PMCID: PMC5965259.
91 Huysmans, Gerard & Radford, Sheena & Brockwell, David & Baldwin, Stephen. (2007). The N-terminal Helix Is a Post-assembly Clamp in the Bacterial Outer Membrane Protein PagP. Journal of molecular biology. 373. 529-40. 10.1016/j.jmb.2007.07.072.
Girolami GS (2024). "Origin and Likely Etymology of the Word 'Trypsin'". Bull. Hist. Chem. 49 (1): 59–60. sighted in wikipeda, tripsis. https://en.wikipedia.org/wiki/Trypsin#cite_note-6
Meuser-Batista M, Corrêa JR, Carvalho VF, de Carvalho Britto CF, Moreira OC, Batista MM, Soares MJ, Filho FA, E Silva PM, Lannes-Vieira J, Silva RC, Henriques-Pons A. Mast cell function and death in Trypanosoma cruzi infection. Am J Pathol. 2011 Oct;179(4):1894-904. doi: 10.1016/j.ajpath.2011.06.014. Epub 2011 Aug 3. PMID: 21819958; PMCID: PMC3181382.
Szempruch AJ, Sykes SE, Kieft R, Dennison L, Becker AC, Gartrell A, Martin WJ, Nakayasu ES, Almeida IC, Hajduk SL, Harrington JM. Extracellular Vesicles from Trypanosoma brucei Mediate Virulence Factor Transfer and Cause Host Anemia. Cell. 2016 Jan 14;164(1-2):246-257. doi: 10.1016/j.cell.2015.11.051. PMID: 26771494; PMCID: PMC4715261.
Stijlemans B, De Baetselier P, Caljon G, Van Den Abbeele J, Van Ginderachter JA, Magez S. Nanobodies As Tools to Understand, Diagnose, and Treat African Trypanosomiasis. Front Immunol. 2017 Jun 30;8:724. doi: 10.3389/fimmu.2017.00724. PMID: 28713367; PMCID: PMC5492476.




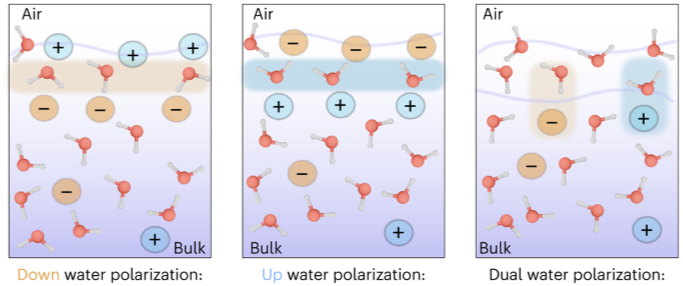

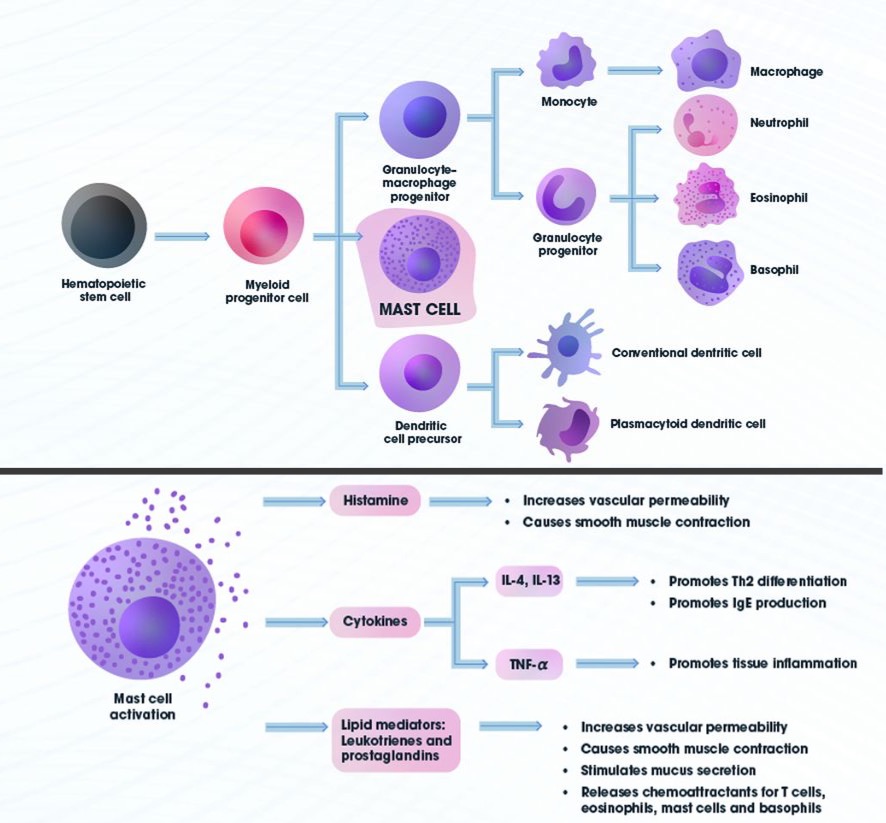



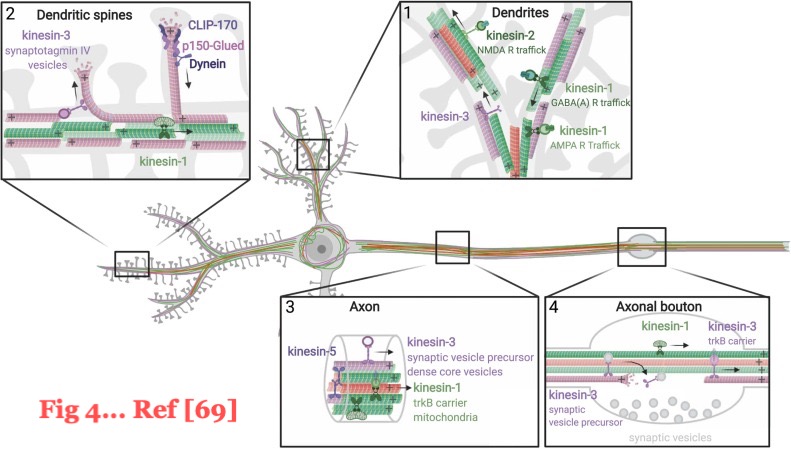









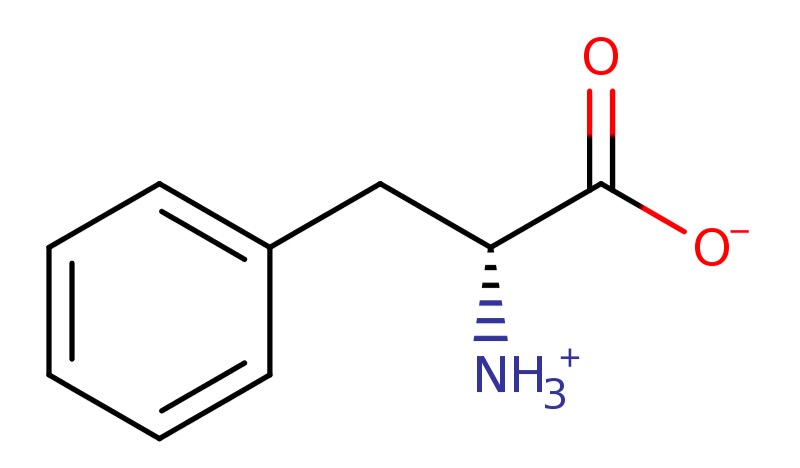

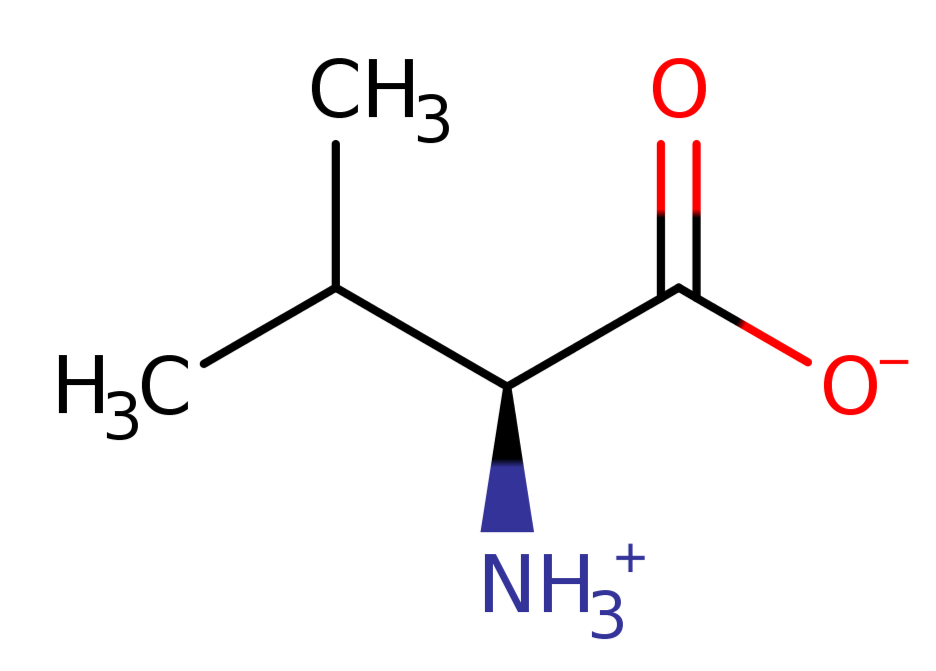

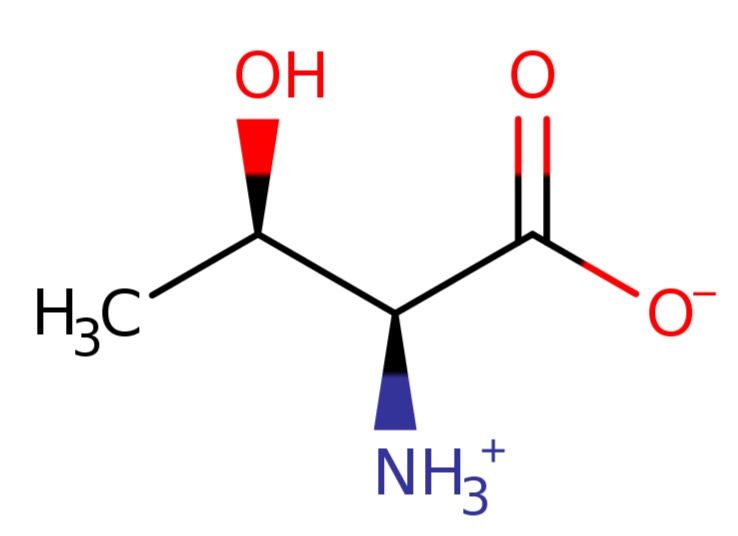



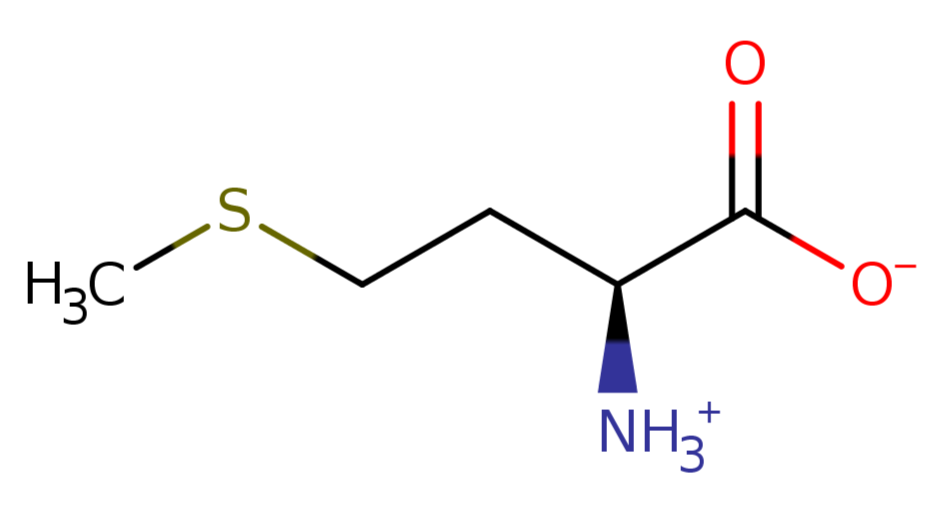

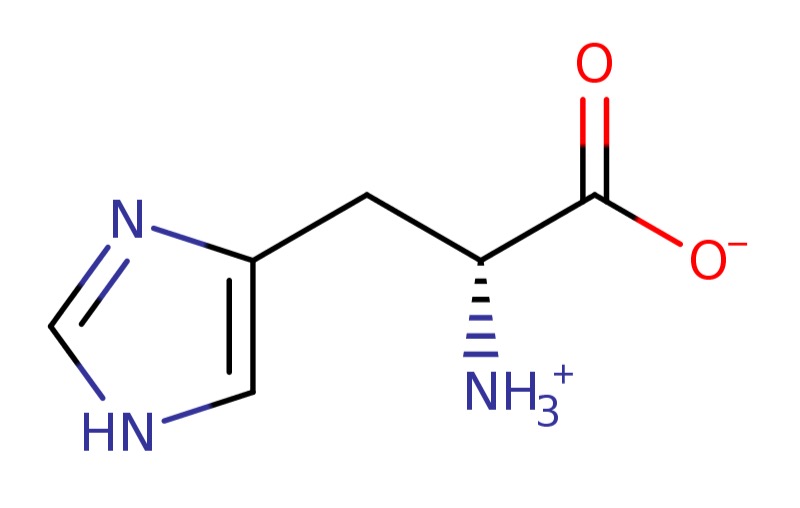

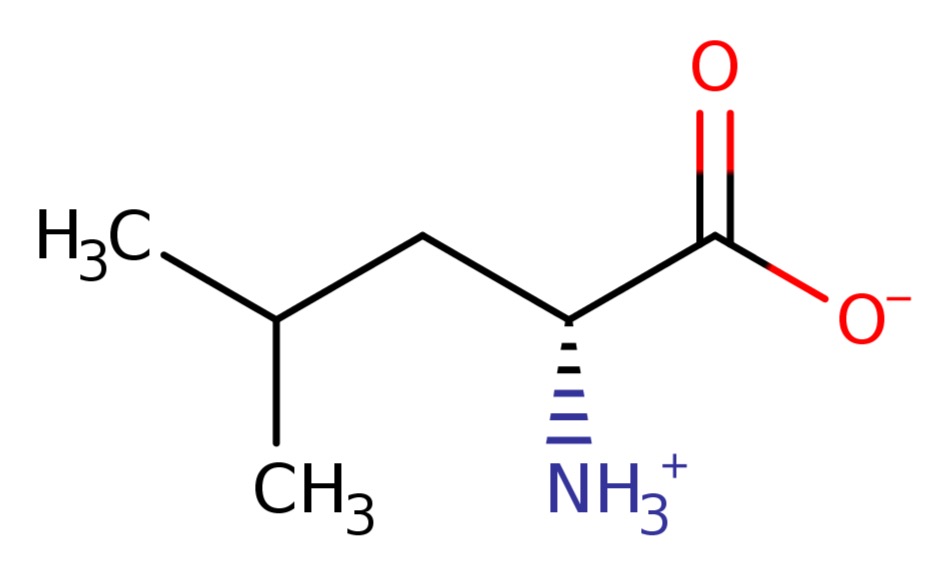

















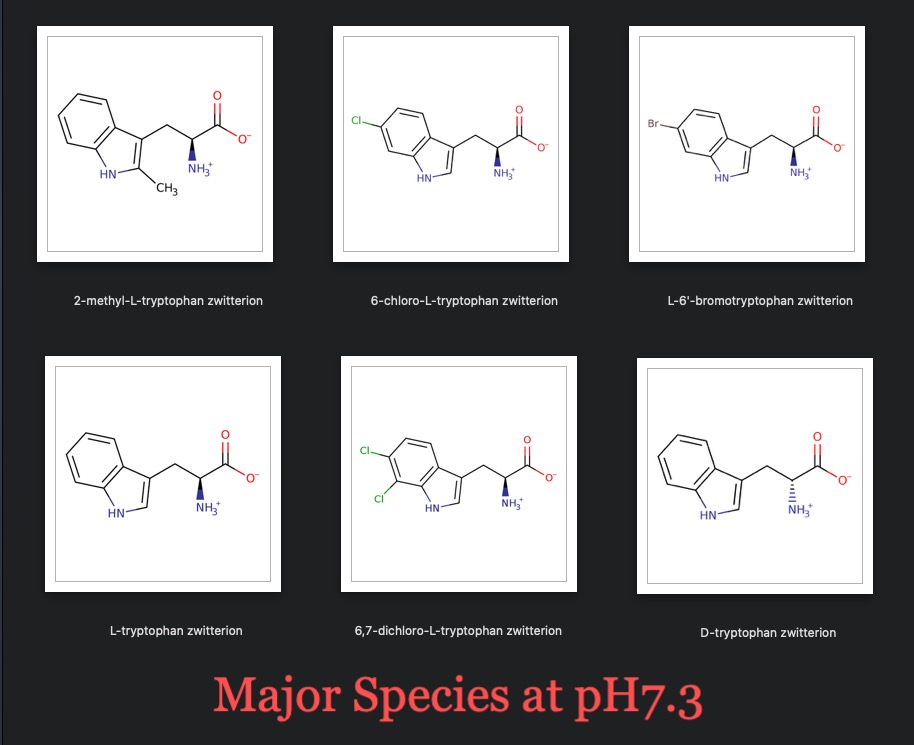



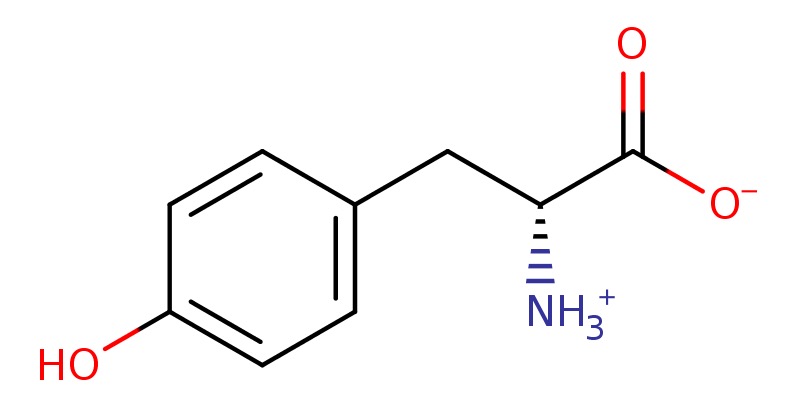

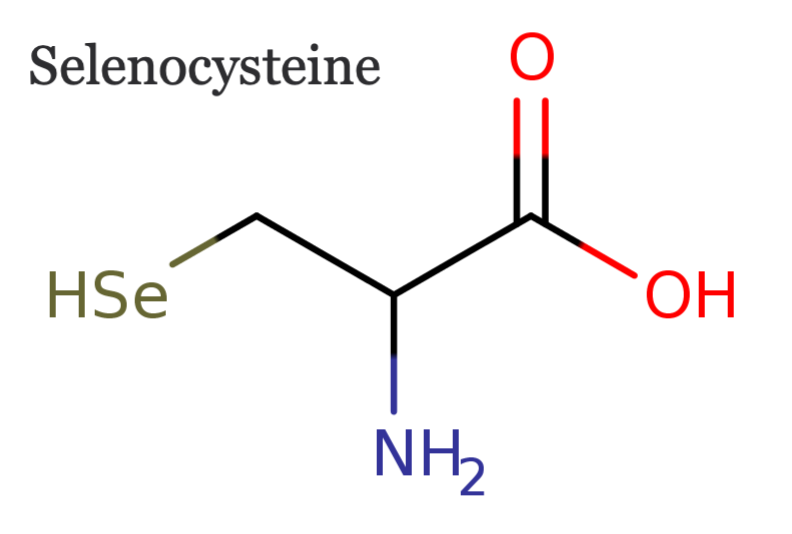





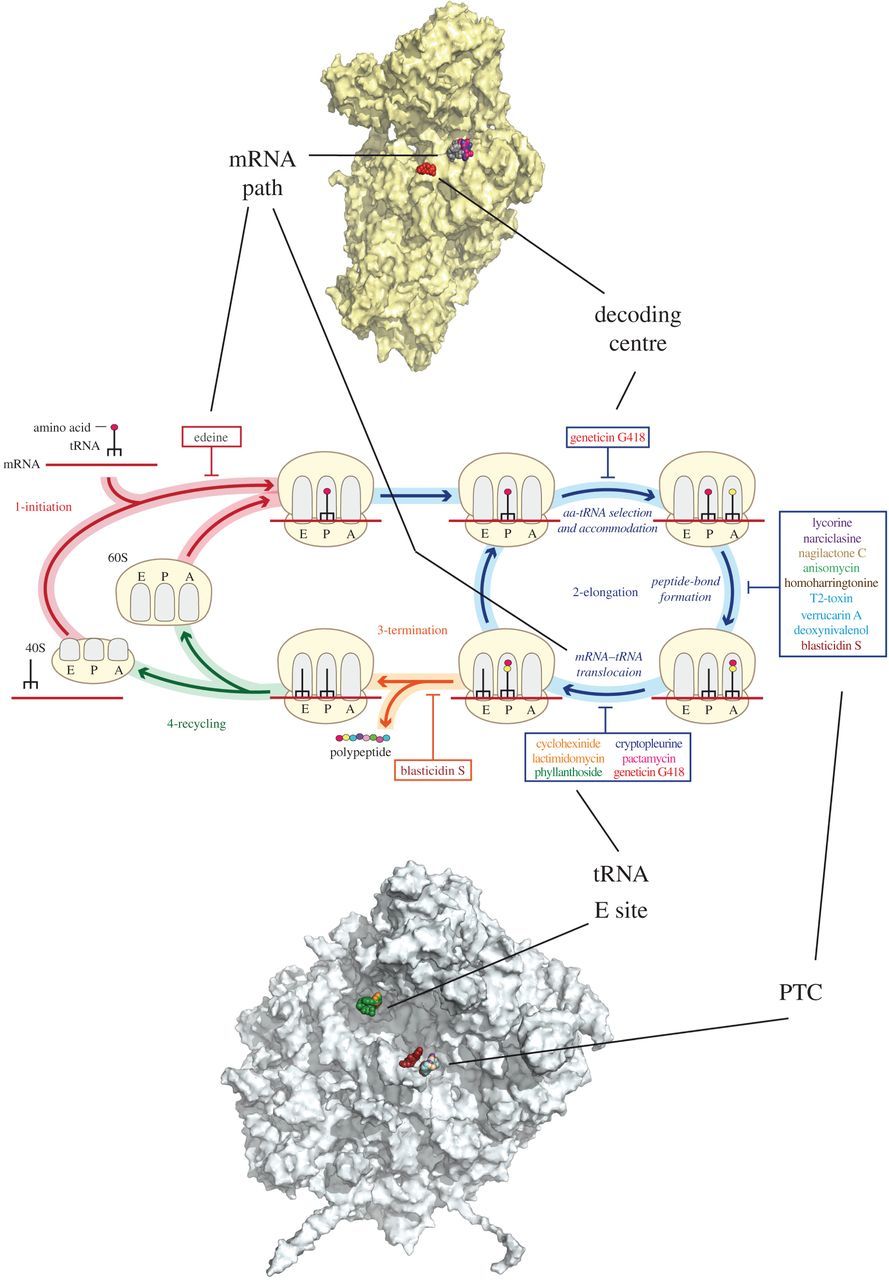


Comments